奥斯卡颗粒中特定 RNA-RNA 相互作用的结构作用
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
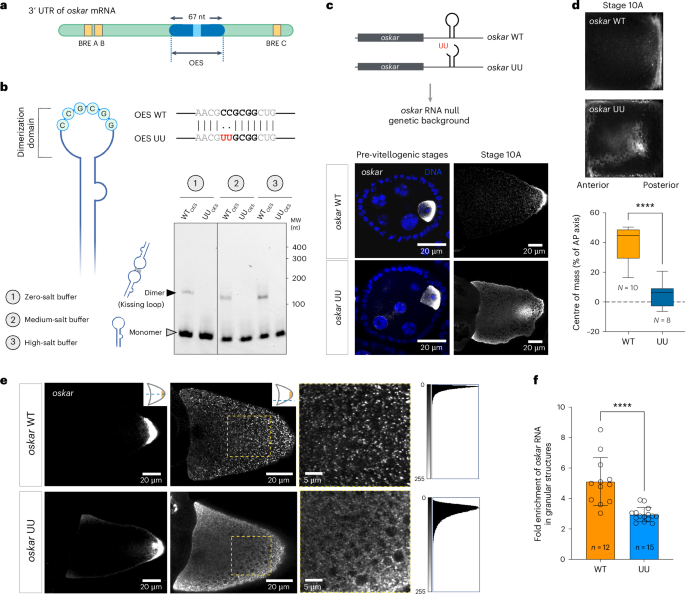
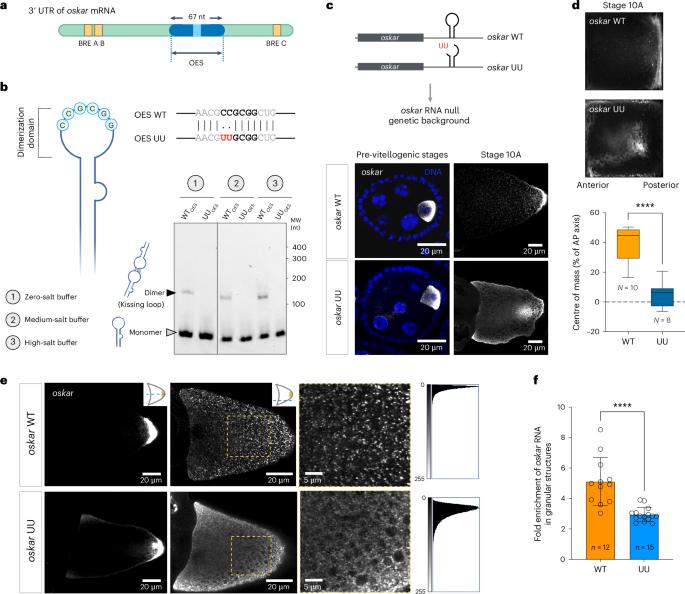
核糖核蛋白(RNP)颗粒是一种无膜凝聚体,通过将特定的 RNA 和蛋白质分隔来组织细胞内空间。研究表明,RNA 可调节 RNA 结合蛋白的相行为,但对体内 RNP 颗粒中分子间 RNA-RNA 相互作用的作用的探索仍然较少。在这里,我们确定了序列特异性 RNA-RNA 吻环相互作用在雌果蝇生殖系中中尺度奥斯卡 RNP 颗粒组装中的作用。我们发现,一个双核苷酸突变破坏了吻合环介导的oskar信使RNA二聚化,从而损害了体外凝集物的形成和发育中卵母细胞中oskar颗粒的组装,导致RNA的后定位缺陷,并在营养胁迫下削弱了oskar相关加工体。这种特异的反式 RNA-RNA 相互作用与支架 RNA 结合蛋白 Bruno 协同作用,推动了凝集体的组装。我们的研究强调了 mRNA 及其特定的二级结构和三级相互作用对形成胚胎发育所必需的 RNP 颗粒的结构性贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An architectural role of specific RNA–RNA interactions in oskar granules
Ribonucleoprotein (RNP) granules are membraneless condensates that organize the intracellular space by compartmentalization of specific RNAs and proteins. Studies have shown that RNA tunes the phase behaviour of RNA-binding proteins, but the role of intermolecular RNA–RNA interactions in RNP granules in vivo remains less explored. Here we determine the role of a sequence-specific RNA–RNA kissing-loop interaction in assembly of mesoscale oskar RNP granules in the female Drosophila germline. We show that a two-nucleotide mutation that disrupts kissing-loop-mediated oskar messenger RNA dimerization impairs condensate formation in vitro and oskar granule assembly in the developing oocyte, leading to defective posterior localization of the RNA and abrogation of oskar-associated processing bodies upon nutritional stress. This specific trans RNA–RNA interaction acts synergistically with the scaffold RNA-binding protein, Bruno, in driving condensate assembly. Our study highlights the architectural contribution of an mRNA and its specific secondary structure and tertiary interactions to the formation of an RNP granule that is essential for embryonic development. Bose, Rankovic et al. show that a specific RNA–RNA kissing-loop interaction plays a crucial role in driving biomolecular condensation of ribonucleoprotein granules in the Drosophila germline.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


