人体类器官药物筛选确定α2-肾上腺素能受体信号传导是软骨再生的治疗靶点
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
在体外和原位引导干细胞向软骨生成方向分化以再生软骨时,会出现脱靶分化和肥大倾向。在这里,我们用携带COL2A1mCherry和COL10A1eGFP双报告基因的人类扩增多能干细胞(hEPSCs)生成了软骨类器官系统,从而实现了对软骨生成和肥大的实时监测。在筛选了 2,040 种美国 FDA 批准的药物后,我们发现α-肾上腺素能受体(α-AR)拮抗剂,尤其是酚妥拉明,能刺激软骨生成,但抑制肥大,而α2-AR 激动剂能减少软骨生成,诱导肥大。酚妥拉明能防止hEPSC软骨类器官模型和人体软骨外植体模型中的软骨退化,并刺激微骨折激活的内源性骨骼干细胞实现透明样软骨再生,而不会出现原位纤维化退化。从机理上讲,α2-AR 信号通过环磷酸鸟苷(cGMP)依赖性分泌型白细胞蛋白酶抑制剂(SLPI)的产生诱导肥大变性。SLPI缺失的软骨类器官具有抗变性能力,有利于大面积软骨缺损的愈合。最终,以α2-AR/SLPI为靶点,通过促进软骨生成和抑制肥大来实现软骨再生,是一种前景广阔、临床可行的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
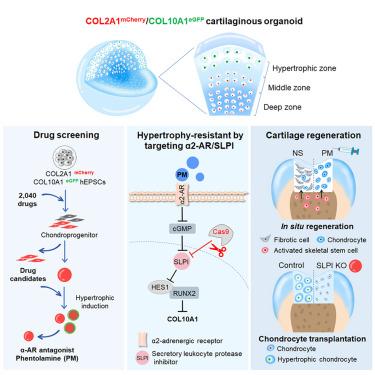
A human organoid drug screen identifies α2-adrenergic receptor signaling as a therapeutic target for cartilage regeneration
Directed differentiation of stem cells toward chondrogenesis in vitro and in situ to regenerate cartilage suffers from off-target differentiation and hypertrophic tendency. Here, we generated a cartilaginous organoid system from human expanded pluripotent stem cells (hEPSCs) carrying a COL2A1mCherry and COL10A1eGFP double reporter, enabling real-time monitoring of chondrogenesis and hypertrophy. After screening 2,040 FDA-approved drugs, we found that α-adrenergic receptor (α-AR) antagonists, especially phentolamine, stimulated chondrogenesis but repressed hypertrophy, while α2-AR agonists reduced chondrogenesis and induced hypertrophy. Phentolamine prevented cartilage degeneration in hEPSC cartilaginous organoid and human cartilage explant models and stimulated microfracture-activated endogenous skeletal stem cells toward hyaline-like cartilage regeneration without fibrotic degeneration in situ. Mechanistically, α2-AR signaling induced hypertrophic degeneration via cyclic guanosine monophosphate (cGMP)-dependent secretory leukocyte protease inhibitor (SLPI) production. SLPI-deleted cartilaginous organoid was degeneration resistant, facilitating large cartilage defect healing. Ultimately, targeting α2-AR/SLPI was a promising and clinically feasible strategy to regenerate cartilage via promoting chondrogenesis and repressing hypertrophy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


