利用基于ctDNA的复发证据开发癌症药物的常设平台
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
开展临床试验所需的时间限制了我们为癌症患者评估和提供新治疗方案的速度。提高试验效率同时保持严谨性的新方法将使患者受益,尤其是在肿瘤学领域,辅助试验有望拦截转移性疾病,但通常需要大量患者和多年时间才能完成。我们设想建立一个常设平台--一个支持对早期癌症治愈性治疗后出现早期疾病分子证据(MED)的癌症患者进行持续识别和试验登记的基础设施,其基础是循环肿瘤 DNA 的存在。MED 可有力地预测随后的复发,绝大多数患者会在 18 个月内出现放射学上的疾病证据。有了这样一个平台,就可以对许多治疗方法进行高效测试,从小型探索性研究到大型关键性试验。招募有 MED 但无放射学疾病证据的患者参加的试验有可能推动药物评估,因为与传统的辅助试验相比,这些试验规模更小(因为复发概率高)、速度更快(因为复发时间短)。循环肿瘤 DNA 也可能为治疗效果提供有价值的早期生物标志物,从而允许进行小型信号搜索试验。在本视角中,我们将讨论如何建立这样一个平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
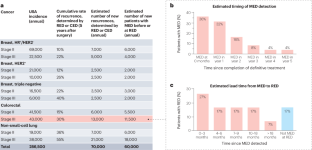
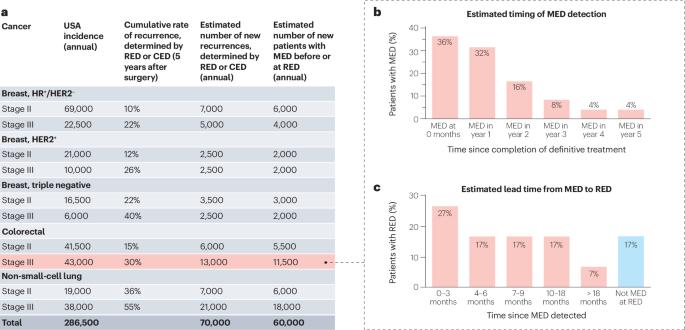
A standing platform for cancer drug development using ctDNA-based evidence of recurrence
The time required to conduct clinical trials limits the rate at which we can evaluate and deliver new treatment options to patients with cancer. New approaches to increase trial efficiency while maintaining rigor would benefit patients, especially in oncology, in which adjuvant trials hold promise for intercepting metastatic disease, but typically require large numbers of patients and many years to complete. We envision a standing platform — an infrastructure to support ongoing identification and trial enrolment of patients with cancer with early molecular evidence of disease (MED) after curative-intent therapy for early-stage cancer, based on the presence of circulating tumour DNA. MED strongly predicts subsequent recurrence, with the vast majority of patients showing radiographic evidence of disease within 18 months. Such a platform would allow efficient testing of many treatments, from small exploratory studies to larger pivotal trials. Trials enrolling patients with MED but without radiographic evidence of disease have the potential to advance drug evaluation because they can be smaller (given high probability of recurrence) and faster (given short time to recurrence) than conventional adjuvant trials. Circulating tumour DNA may also provide a valuable early biomarker of treatment effect, which would allow small signal-finding trials. In this Perspective, we discuss how such a platform could be established. Efficient clinical trials are crucial for advancing cancer care. In this Perspective, the authors propose a platform that leverages circulating tumour DNA to identify patients with early-stage cancer at high risk of recurrence and enrol them onto therapeutic clinical trials. This approach would enable faster, smaller trials and streamline evaluation of drugs aimed at preventing disease recurrence and increasing cure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


