使电催化寿命合理化的微动力学模型:活性与稳定性之间的权衡
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
要想以环保方式生产氢气和商品化学品,就必须使用可运行数年的电催化剂。然而,由于缺乏定量预测催化寿命的概念框架,设计具有长工作寿命的材料具有挑战性。在此,我们报告了一个微动力学方程,该方程可量化正在发生溶解的电催化剂的寿命。该方程是利用催化作用比失活快得多这一事实,通过准稳态近似法求解常微分方程而得到的。所有化学反应都被模拟为不可逆的一阶基本反应。根据这一假设,催化速率与失活速率呈线性相关,从而导致活性与稳定性之间的权衡关系。氧化锰电催化剂在氧进化反应中的理论寿命和实验寿命之间的相关性(r2 = 0.86)支持了我们的模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
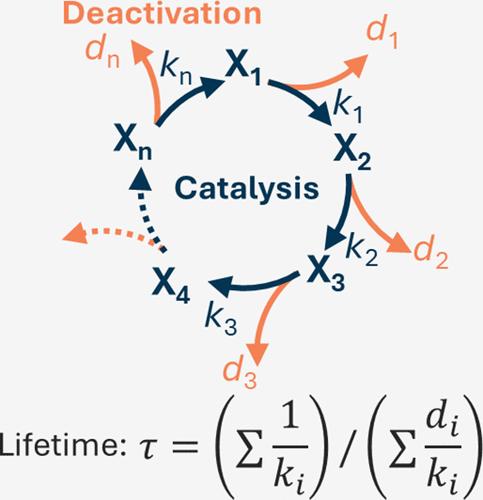
Microkinetic Model to Rationalize the Lifetime of Electrocatalysis: Trade-off between Activity and Stability
Electrocatalysts which can operate for several years are required to produce hydrogen and commodity chemicals in an environmentally friendly manner. However, designing materials with long operational lifetimes is challenging, due to the lack of a conceptual framework to predict catalytic lifetimes quantitatively. Here, we report a microkinetic equation which quantifies the lifetime of an electrocatalyst undergoing dissolution. This equation was obtained by taking advantage of the fact that catalysis is much faster than deactivation, which allows the ordinary differential equations to be solved via the quasi steady-state approximation. All chemical reactions were modeled as irreversible, first-order elementary reactions. Under this assumption, the catalytic rate correlates linearly with the deactivation rate, leading to a trade-off relationship between activity and stability. Our model was supported by the correlation between theoretical and experimental lifetimes (r2 = 0.86) of a manganese oxide electrocatalyst during the oxygen evolution reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


