苯基迁移介导的多孔石墨烯纳米带的表面合成
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
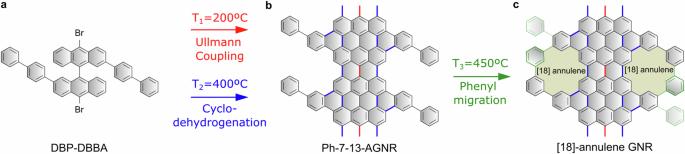
创新前驱体设计和反应类型的引入推动了原子精度石墨烯纳米结构的表面合成技术的进步。迄今为止,后者仅限于涉及特定原子或基团裂解的交叉耦合和环化反应。在本文中,我们阐明了如何利用附着在石墨烯纳米带上的苯基取代基的迁移,在纳米结构的边缘生成[18]-annulene 孔阵列。利用键分辨扫描隧道显微镜和非线性计算技术进行的一项综合研究揭示了这一顺序途径。通过将石墨烯纳米带锚定在临近表面的台阶上,孔隙形成的产率达到了最大化,凸显了这些基底引导反应路径的潜力。我们的研究为表面合成工具箱引入了一种新的反应以及一条连续路线,从而使这一策略能够扩展到其他多孔纳米结构的形成。石墨烯纳米带的表面合成通常依赖于乌尔曼聚合,然后是内部环氢化。在这里,作者在这两个步骤之后扩展了合成方案,增加了受控苯基迁移和带内芳基-芳基脱氢偶联,从而得到了边缘具有周期性[18]环烯孔阵列的石墨烯纳米带。本文章由计算机程序翻译,如有差异,请以英文原文为准。
On-surface synthesis of porous graphene nanoribbons mediated by phenyl migration
Advancements in the on-surface synthesis of atomically precise graphene nanostructures are propelled by the introduction of innovative precursor designs and reaction types. Until now, the latter has been confined to cross-coupling and cyclization reactions that involve the cleavage of specific atoms or groups. In this article, we elucidate how the migration of phenyl substituents attached to graphene nanoribbons can be harnessed to generate arrays of [18]-annulene pores at the edges of the nanostructures. This sequential pathway is revealed through a comprehensive study employing bond-resolved scanning tunneling microscopy and ab-initio computational techniques. The yield of pore formation is maximized by anchoring the graphene nanoribbons at steps of vicinal surfaces, underscoring the potential of these substrates to guide reaction paths. Our study introduces a new reaction to the on-surface synthesis toolbox along with a sequential route, altogether enabling the extension of this strategy towards the formation of other porous nanostructures. The on-surface synthesis of graphene nanoribbons typically relies on Ullmann polymerization followed by an internal cyclodehydrogenation. Here, following these two steps, the authors expand the synthetic protocol by adding controlled phenyl migration and intraribbon aryl-aryl dehydrogenative coupling to afford graphene nanoribbons with periodic arrays of [18]annulene pores at the edges.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


