将多柔比星和 PD-L1 siRNA 共同封装在双反应共价有机框架中,实现协同的癌症化学免疫疗法
IF 5.1
Q1 POLYMER SCIENCE
引用次数: 0
摘要
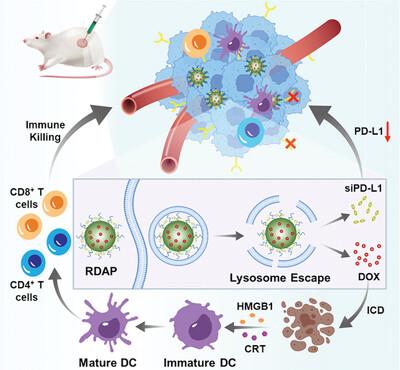
化疗免疫疗法将化疗药物与免疫调节剂结合在一起,已成为一种很有前景的方法,可改善免疫疗法普遍不佳的临床效果。然而,如何协调与激活抗肿瘤免疫反应的不同机制相关的多个事件是一项重大挑战。本文介绍了一种利用双响应共价有机框架(COFs)共同包被多柔比星(DOX)和程序性细胞死亡配体-1(PD-L1)siRNA(siPD-L1)(称为 RDAP)来协同化疗免疫疗法的新方法。在该系统中,用聚-L-赖氨酸(PLL)修饰的 COFs 可在其多孔结构中吸附 DOX,并通过静电作用将 siPD-L1 结合到其表面。由此形成的 RDAP 配方可通过 "质子海绵效应 "逃逸出溶酶体,并在偶氮还原酶的作用下破裂,从而将 DOX 和 siPD-L1 有效释放到 4T1 细胞的细胞质中。RDAP 含有双重治疗药物,能有效清除肿瘤细胞,并通过 DOX 引发显著的免疫原性细胞死亡(ICD)。此外,它还能同时抑制肿瘤细胞中 PD-L1 的表达,从而显著增强抗肿瘤免疫反应,协同提高抗肿瘤疗效。在小鼠模型中,RDAP 在消除原发肿瘤和远处肿瘤方面表现出卓越的疗效,同时对 4T1 小鼠乳房肺转移瘤具有明显的抗转移效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Copackaging Doxorubicin and PD-L1 siRNA in Dual-Responsive Covalent Organic Frameworks for Synergistic Cancer Chemoimmunotherapy
Chemoimmunotherapy, which integrates chemotherapeutic drugs with immune modulators, has emerged as a promising approach to enhance the generally lackluster clinical outcomes of immunotherapy. Nevertheless, a significant challenge lies in orchestrating multiple events associated with diverse mechanisms that activate antitumor immune responses. Here, a novel approach utilizing dual-responsive covalent organic frameworks (COFs) co-encapsulating doxorubicin (DOX) and programmed cell death ligand-1 (PD-L1) siRNA (siPD-L1), termed RDAP, is presented for synergistic chemoimmunotherapy. In this system, COFs modified with poly-L-lysine (PLL) can adsorb DOX within their porous structure and bind siPD-L1 onto their surface via electrostatic interactions. The resulting RDAP formulation can escape from lysosome via the “proton sponge effect” and undergo rupture upon exposure to azoreductase, leading to the efficient release of DOX and siPD-L1 into the cytoplasm of 4T1 cells. The RDAP, harboring dual therapeutic agents, can effectively eliminate tumor cells and trigger significant immunogenic cell death (ICD) through DOX. Additionally, it concomitantly suppresses PD-L1 expression in tumor cells, thereby significantly enhancing the antitumor immune response and resulting in synergistically improved antitumor efficacy. In mouse models, RDAP demonstrated exceptional efficacy in eliminating both primary and distant tumors, along with a pronounced antimetastatic effect against 4T1 murine breast-to-lung metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


