操纵锌沉积行为,通过高价锆离子实现高倍率锌水电池
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
锌离子水电池是一种具有高安全性和低成本的优秀储能设备。然而,锌阳极表面发生的腐蚀反应和锌枝晶的形成阻碍了其进一步发展。为了解决这些问题,人们使用醋酸锆(ZA)作为 ZnSO4 电解液的电解质添加剂。由于 Zr4+ 比 Zn2+ 具有更高的正电性,这些高价金属阳离子会优先吸附在金属锌表面,从而屏蔽锌与电解液之间的寄生反应,重塑电场分布,并引导 Zn 离子优先均匀沉积在 Zn (002) 晶面上。此外,电化学循环后 Zr4+ 在金属锌上的吸附可增强锌原子扩散的能量势垒,从而产生较高的抗腐蚀性并操纵 Zn2+ 的成核构型。由于这些特性,添加了ZA电解质的Zn//Zn对称电池能够在40 mA cm-2的超高电流密度下循环400小时,面积容量为2 mAh cm-2。同时,MnO2//Zn 纽扣电池在 1 A g-1 的电流密度下循环 850 次后,仍有 81.7 mAh g-1(容量保持率 85%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
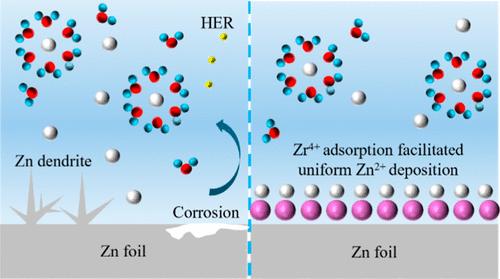
Manipulation of Zn Deposition Behavior to Achieve High-Rate Aqueous Zinc Batteries via High Valence Zirconium Ions
Aqueous zinc ion batteries are excellent energy storage devices with high safety and low cost. However, the corrosion reaction and zinc dendrite formation occurring on the surface of zinc anodes are hindering their further development. To solve the problems, zirconium acetate (ZA) was used as an electrolyte additive in the ZnSO4 electrolyte. Attributing to the higher electro-positivity of Zr4+ than Zn2+, these high valence metal cations preferentially adsorb onto the surface of metallic zinc, shielding parasitic reactions between zinc and electrolyte, reshaping the electric field distribution, and directing preferential homogeneous deposition of Zn-ions on the Zn (002) crystal plane. Furthermore, the adsorption of Zr4+ on the Zn metal after electrochemical cycles can enhance the energy barrier of zinc atom diffusion, resulting in high resistance of corrosion and manipulation of the Zn2+ nucleation configuration. Attributing to these properties, the Zn//Zn symmetric cell with an electrolyte additive of ZA was able to cycle for 400 h under an extremely high current density of 40 mA cm–2 with an area capacity of 2 mAh cm–2. Meanwhile, the MnO2//Zn coin cell still had 81.7 mAh g–1 (85% retention of capacity) after 850 cycles under a current density of 1 A g–1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


