通过锂/镍无序调节提高富锂层状氧化物阴极的阳离子和阴离子氧化还原活性
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
富锂层状氧化物(LLOs)作为下一代高能量密度锂离子电池(LIBs)的正极材料,越来越受到人们的认可。然而,由于不可逆 O 释放导致的严重结构退化,它们存在电压衰减和初始库仑效率(ICE)低的问题。在此,我们引入了一种三合一策略,即增加镍和锰的含量,同时进行锂/镍无序化和 TM-O 共价调节,以同时提高阳离子和阴离子氧化还原活性,从而增强 LLO 的电化学活性。目标材料 Li1.2Ni0.168Mn0.558Co0.074O2 (L1) 的 ICE 提高了 87.2%,比容量达到 293.2 mA h g-1,电压衰减小于 0.相比之下,Li1.2Ni0.13Mn0.54Co0.13O2(Ls)的初始容量为 274.4 mA h g-1,ICE 为 73.8%,在 1C 下循环 300 次的电压衰减为 0.84 mV/周期。理论计算显示,L1 在费米能级附近的状态密度(DOS)区域大于 Ls,这表明其阴离子和阳离子氧化还原反应活性高于 Ls。此外,由于 L1 的锂/镍无序度更高,达到 4.76%(通过 X 射线衍射 Rietveld 精炼量化),且 TM-O 共价性增强,L1 显示出更高的 O 空位形成能,使晶格 O 更难释放,从而提高了电化学稳定性。此外,Li/Ni 无序度的增加还导致 Li 层中存在更多的 Ni2+,在 Li+ 脱嵌过程中起到支柱作用,从而提高了结构的稳定性。这项研究不仅为设计具有更高容量和 ICE 的低 Co LLO 提供了一种可行的方法,而且极大地促进了对高性能 LIB 阴极结构调节的基本理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
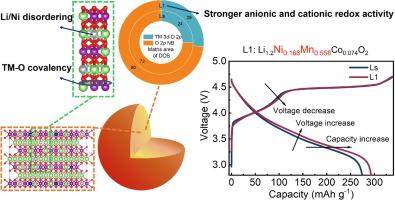
Boosting cationic and anionic redox activity of Li-rich layered oxide cathodes via Li/Ni disordered regulation
Lithium-rich layered oxides (LLOs) are increasingly recognized as promising cathode materials for next-generation high-energy-density lithium-ion batteries (LIBs). However, they suffer from voltage decay and low initial Coulombic efficiency (ICE) due to severe structural degradation caused by irreversible O release. Herein, we introduce a three-in-one strategy of increasing Ni and Mn content, along with Li/Ni disordering and TM–O covalency regulation to boost cationic and anionic redox activity simultaneously and thus enhance the electrochemical activity of LLOs. The target material, Li1.2Ni0.168Mn0.558Co0.074O2 (L1), exhibits an improved ICE of 87.2% and specific capacity of 293.2 mA h g−1 and minimal voltage decay of less than 0.53 mV cycle−1 over 300 cycles at 1C, compared to Li1.2Ni0.13Mn0.54Co0.13O2 (Ls) (274.4 mA h g−1 for initial capacity, 73.8% for ICE and voltage decay of 0.84 mV/cycle over 300 cycles at 1C). Theoretical calculations reveal that the density of states (DOS) area near the Fermi energy level for L1 is larger than that of Ls, indicating higher anionic and cationic redox reactivity than Ls. Moreover, L1 exhibits increased O-vacancy formation energy due to higher Li/Ni disordering of 4.76% (quantified by X-ray diffraction Rietveld refinement) and enhanced TM–O covalency, making lattice O release more difficult and thus improving electrochemical stability. The increased Li/Ni disordering also leads to more Ni2+ presence in the Li layer, which acts as a pillar during Li+ de-embedding, improving structural stability. This research not only presents a viable approach to designing low-Co LLOs with enhanced capacity and ICE but also contributes significantly to the fundamental understanding of structural regulation in high-performance LIB cathodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


