CaH2 促进碳酸镍界面的二氧化碳甲烷化活性
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
过渡金属-碳酸盐界面通常是异相催化反应中的活性位点。在二氧化碳氢化反应中,过渡金属与金属碳酸盐之间的界面表现出动态平衡,包括表面碳酸盐氢化和二氧化碳化学吸附。然而,关于过渡金属与碱土金属碳酸盐界面活性工程化用于催化二氧化碳转化的报道却很少。这项研究表明,在 Ni/CaCO3 中加入 CaH2 可提高催化剂的二氧化碳甲烷化活性。在 400 °C 时,Ni/CaH2-CaCO3 的二氧化碳转化率达到 68.5%,远高于 Ni/CaCO3 催化剂(31.6%)和 Ni/CaH2-CaO 催化剂(42.4%)。此外,Ni/CaH2-CaCO3 催化剂在 400 °C 和 8 巴条件下进行 24 小时稳定性测试时仍保持稳定。我们的研究表明,CaH2 在促进碳酸化镍界面的二氧化碳甲烷化活性方面发挥了关键作用。CaH2 可以改变 Ni 的电子结构并调整 CaCO3 的结构特性,从而产生中等碱性位点(OH 基团),有利于 H2 和 CO2 的活化。原位傅立叶变换红外光谱(FTIR)分析结合密度泛函理论计算证明,二氧化碳活化发生在 CaH2 改性碳酸镍表面的羟基(OH)上,从而形成 CO3H* 物种。此外,我们的研究还证实,二氧化碳在 Ni/CaH2-CaCO3 催化剂上的甲烷化是通过甲酸途径进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
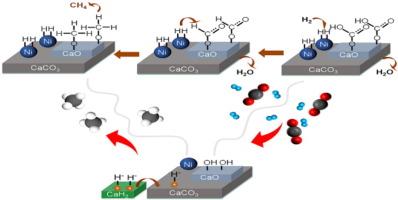
CaH2-promoted activity of Ni-carbonate interface for CO2 methanation
Transition metal-carbonate interfaces often act as active sites in heterogeneous catalytic reactions. The interface between transition metal and metal carbonate exhibits a dynamic equilibrium during the CO2 hydrogenation reaction, involving surface carbonate hydrogenation and CO2 chemisorption. Nonetheless, there have been few reports on engineering the activity of the interface between transition metal and alkaline earth metal carbonate for catalytic CO2 conversion. This work demonstrated that the incorporation of CaH2 in Ni/CaCO3 enhances the CO2 methanation activity of the catalysts. The CO2 conversion for Ni/CaH2-CaCO3 reached 68.5% at 400 °C, which was much higher than that of the Ni/CaCO3 (31.6%) and Ni/CaH2-CaO (42.4%) catalysts. Furthermore, the Ni/CaH2-CaCO3 catalysts remained stable during the stability test for 24 h at 400 °C and 8 bar. Our research revealed that CaH2 played a crucial role in promoting the activity of the Ni-carbonate interface for CO2 methanation. CaH2 could modify the electronic structure of Ni and tune the structural properties of CaCO3 to generate medium basic sites (OH groups), which are favorable for the activation of H2 and CO2. In-situ Fourier transform infrared spectroscopy (FTIR) analysis combined with density functional theory calculations demonstrated that CO2 activation occurs at the hydroxyl group (OH) on the CaH2-modified Ni-carbonate surface, leading to the formation of CO3H* species. Furthermore, our study has confirmed that CO2 methanation over the Ni/CaH2-CaCO3 catalysts proceeds via the formate pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


