在现实条件下增强电化学硝酸盐还原的电催化剂原位进化
IF 14.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
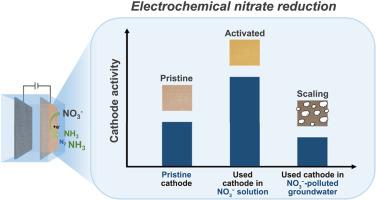
电化学硝酸盐还原成氨(ENRA)因其在水质修复和可持续氨生产方面的潜力而备受关注,它为高能耗的哈伯-博什工艺提供了一种更环保的替代方法。目前有关 ENRA 的研究致力于利用复杂的催化剂提高氨的选择性和生产率。然而,人们对 ENRA 的性能以及催化活性在更复杂溶液(如硝酸盐污染的地下水)中的变化知之甚少。在此,我们首先利用商用阴极探讨了 Ca2+ 和碳酸氢盐对 ENRA 的影响。我们发现,使用过的镍或铜泡沫阴极的催化活性明显优于原始阴极,这是因为在 ENRA 过程中,使用过的阴极上出现了新的催化物种。相比之下,非活性 Ti 或 Sn 阴极的硝酸盐转化性能受 Ca2+ 或碳酸氢盐的影响较小,因为它们原本的活性较低。此外,Ca2+ 和碳酸氢盐的共存会在原位形成的活性位点上形成鳞片(CaCO3),从而抑制硝酸盐的转化。同样,由于存在硬度离子和可能的有机物质,ENRA 在处理实际地下水时容易出现性能快速下降的问题,因为这些物质会迅速阻塞硝酸盐还原的活性位点。我们的研究表明,要确保 ENRA 在处理受硝酸盐污染的天然水体时的长期稳定性,并在更现实的条件下利用 ENRA 的环境相关性,还需要做更多的工作。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In situ evolution of electrocatalysts for enhanced electrochemical nitrate reduction under realistic conditions
Electrochemical nitrate reduction to ammonia (ENRA) is gaining attention for its potential in water remediation and sustainable ammonia production, offering a greener alternative to the energy-intensive Haber-Bosch process. Current research on ENRA is dedicated to enhancing ammonia selectively and productivity with sophisticated catalysts. However, the performance of ENRA and the change of catalytic activity in more complicated solutions (i.e., nitrate-polluted groundwater) are poorly understood. Here we first explored the influence of Ca2+ and bicarbonate on ENRA using commercial cathodes. We found that the catalytic activity of used Ni or Cu foam cathodes significantly outperforms their pristine ones due to the in situ evolution of new catalytic species on used cathodes during ENRA. In contrast, the nitrate conversion performance with nonactive Ti or Sn cathode is less affected by Ca2+ or bicarbonate because of their original poor activity. In addition, the coexistence of Ca2+ and bicarbonate inhibits nitrate conversion by forming scales (CaCO3) on the in situ-formed active sites. Likewise, ENRA is prone to fast performance deterioration in treating actual groundwater over continuous flow operation due to the presence of hardness ions and possible organic substances that quickly block the active sites toward nitrate reduction. Our work suggests that more work is required to ensure the long-term stability of ENRA in treating natural nitrate-polluted water bodies and to leverage the environmental relevance of ENRA in more realistic conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Science and Ecotechnology
Multiple-
CiteScore
20.40
自引率
6.30%
发文量
11
审稿时长
18 days
期刊介绍:
Environmental Science & Ecotechnology (ESE) is an international, open-access journal publishing original research in environmental science, engineering, ecotechnology, and related fields. Authors publishing in ESE can immediately, permanently, and freely share their work. They have license options and retain copyright. Published by Elsevier, ESE is co-organized by the Chinese Society for Environmental Sciences, Harbin Institute of Technology, and the Chinese Research Academy of Environmental Sciences, under the supervision of the China Association for Science and Technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


