苯氧乙酰氯对菱镁矿的选择性抑制:从白云石中有效浮选分离菱镁矿的意义
IF 4.9
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
为了有效提高菱镁矿与白云石的浮选分离效果,首先引入了有机调节剂苯氧乙酰氯(POC)作为菱镁矿的选择性抑制剂,并以十二胺(DDA)作为捕收剂。单矿物浮选结果表明,POC 能选择性地抑制菱镁矿,但几乎不影响白云石。菱镁矿和白云石合成混合物的反浮选结果与单矿物浮选结果基本一致。以 DDA 作为捕收剂,以 POC 作为抑制剂,白云石选择性地浮选菱镁矿。在 pH 值为 8.00 时,采用 0.50 mg/mL POC 和 30.00 mg/L DDA 的试剂方案可有效分离菱镁矿和白云石。当合成矿物的混合矿石氧化镁含量为 42.60 %、氧化钙含量为 2.84 %时,可获得氧化镁含量为 46.85 %、氧化钙含量为 0.45 %的菱镁矿精矿。接触角、Zeta 电位、傅立叶变换红外光谱(FTIR)、X 射线光电子能谱(XPS)和 DFT 分析表明,POC 通过在菱镁矿表面螯合镁,选择性地吸附在菱镁矿表面。接触角测试结果表明,在 DDA 系统中,POC 选择性地降低了菱镁矿的表面疏水性。此外,Zeta 电位测量和傅立叶变换红外分析表明,在 DDA 之前添加 POC 对 DDA 在白云石上的吸附没有显著影响;而添加 POC 则强烈阻止了 DDA 在菱镁矿上的吸附,导致两种矿物的浮选性能存在显著差异。此外,XPS 分析和 DFT 计算证实,POC 在菱镁矿表面的吸附作用可能是由于 POC 富电子基团与暴露在菱镁矿中的镁之间的相互作用,POC 可用作菱镁矿浮选的高性能抑制剂,实现脱钙。根据这些实验结果,提出了菱镁矿浮选分离过程的可能模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
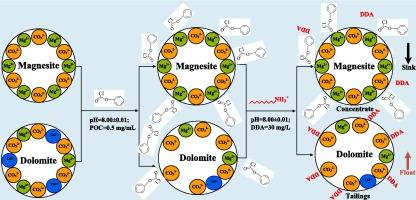
Selective depression of phenoxyacetyl chloride on magnesite: Implications for effective flotation separation of magnesite from dolomite
In order to improve the flotation separation of magnesite from dolomite effectively, the organic regulator, phenoxyacetyl chloride (POC), was first introduced as a selective depressant of magnesite, with dodecylamine (DDA) as the collector. The results of single mineral flotation showed that POC could selectively inhibit magnesite but hardly affect dolomite. The results of reverse flotation of a synthetic mixture of magnesite and dolomite were basically consistent with those of single mineral flotation. With DDA as the collector and POC as the depressant, dolomite floated selectively against magnesite. Magnesite and dolomite could be efficiently separated at pH 8.00 with a reagent scheme of 0.50 mg/mL POC and 30.00 mg/L DDA. When the mixed ore for the synthetic mineral, grading 42.60 % MgO and 2.84 % CaO, a magnesite concentrate with 46.85 % MgO and 0.45 % CaO was achieved. Contact angle, Zeta potential, Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and DFT analyses showed that POC selectively adsorbed on the surface of magnesite, by chelating Mg on the surface of magnesite. The results from the contact angle tests indicated that POC selectively reduced the surface hydrophobicity of magnesite in the DDA system. Besides, Zeta-potential measurements and FTIR analyses revealed that the addition of POC prior to DDA had no significant impact on the adsorption of DDA onto dolomite; the addition of POC strongly prevented DDA from being adsorbed onto magnesite, resulting in a significant difference in the flotation performances of the two minerals. Furthermore, XPS analyses and DFT calculations confirmed that the adsorption of POC on the magnesite surface could be attributed to the interaction between the POC electron-rich groups and the Mg exposed to magnesite, POC could be used as a high-performance inhibitor for the magnesite flotation to realize the decalcificationic. Based on these experimental results, a possible model for the flotation separation process of magnesite has been proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


