从嗜硫红藻中回收细胞外 RNA 的新方法
IF 2.5
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
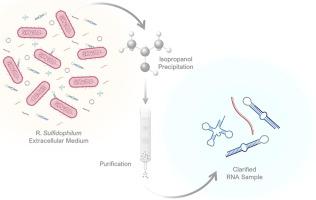
由于有可能开发出可行而有效的疗法,人们对基于 RNA 的药物的开发十分关注。然而,将其转化为临床实践在很大程度上依赖于高效的技术,以生产大量高纯度和高质量的这些生物大分子。RNA 通常是通过化学或酶法生产的,具有这些局限性。从这个意义上说,重组生产是一种有前途、成本效益高的方法,可以大规模生产 RNA。嗜硫红藻(Rhodovulum sulfidophilum,R. sulfidophilum)是一种海洋紫色细菌,其优势是将 RNA 分泌到细胞外培养基中,而细胞外培养基中的 RNase 和其他杂质含量较低。因此,与涉及细胞裂解的标准提取方案相比,RNA 的回收可以得到简化,从而获得更清晰的样品并改进下游工艺。在这项工作中,用编码前 miR-29b-1 的质粒 DNA 转化了嗜硫杆菌,已知前 miR-29b-1 参与了阿尔茨海默病的发病途径。生产完成后,通过不同的提取方法回收 pre-miR-29b-1,以验证最有利的提取方法。随后确立了一种细胞外 RNA 回收方案,结果显示这是一种更简单、耗时更少的方法,与其他已有方法相比,可减少约 16 小时的执行时间和所需试剂的数量。新策略的另一个优势是,它消除了细胞内 RNA 提取过程中常规使用的有毒有机化合物。总之,本文所述的优化流程使用异丙醇作为沉淀剂,为重组 RNA 的回收和浓缩提供了一种更环保、更安全、更快速的替代方法,细胞外 RNA 回收率为 7 μg/mL,目标 pre-miRNA-29b-1 回收率为 0.7 μg/L 培养基。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A new approach for extracellular RNA recovery from Rhodovulum sulfidophilum
The development of RNA-based drugs is highly pursued due to the possibility of creating viable and effective therapies. However, their translation to clinical practice strongly depends on efficient technologies to produce substantial levels of these biomolecules, with high purity and high quality. RNAs are commonly produced by chemical or enzymatic methods, displaying these limitations. In this sense, recombinant production arises as a promising, cost-effective method, allowing large-scale production of RNA. Rhodovulum sulfidophilum (R. sulfidophilum), a marine purple bacterium, offers the advantage of RNA secretion into the extracellular medium, which contains low levels of RNases and other impurities. Therefore, RNA recovery can be simplified compared to standard extraction protocols involving cell lysis, resulting in a more clarified sample and an improved downstream process. In this work, R. sulfidophilum was transformed with a plasmid DNA encoding pre-miR-29b-1, which is known to be involved in the Alzheimer's disease pathway. After production, the pre-miR-29b-1 was recovered through different extraction methods to verify the most advantageous one. A protocol for extracellular RNA recovery was then established, revealing to be a simpler and less time-consuming method, reducing around 16 h in execution time and the quantity of reagents needed when compared to other established methods. The new strategy brings the additional advantage of eliminating the toxic organic compounds routinely used in intracellular RNA extractions. Overall, the optimized process described here, using isopropanol as the precipitation agent, offers a greener, safer, and faster alternative for recombinant RNA recovery and concentration, with an extracellular RNA recovery of 7 μg/mL and target pre-miRNA-29b-1 recovery of 0.7 μg/L of medium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


