超越基础:具有可变总允许误差 (TEa) 的检验医学中的西格玛评分
IF 3.2
3区 医学
Q2 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
摘要
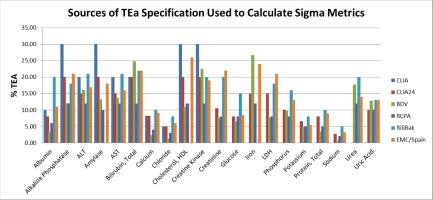
背景为了诊断疾病、跟踪治疗效果并做出明智的临床决策,医生们依赖于实验室的结果。准确和精确的结果可最大限度地减少额外检测的必要性,从而节省时间和金钱,同时提高患者满意度。内部质量控制和外部质量保证计划(EQAS)是用于评估临床实验室绩效的指标。西格玛指标是众多质量指标之一,可用来衡量误差的大小。要计算西格玛分数,需要偏差率、CV% 和允许总误差 (TEa)。允许总误差(TEa)是一个重要的基准,它规定了某种分析物偏离目标值的允许限度。然而,目前还没有就确定 TEa 目标达成适当的共识,也没有充分确定这一限制因素在标准实验室实践和西格玛计算中的影响。选择正确的允许总误差(TEa)目标是采用西格玛度量法时面临的最大挑战之一,因为根据不同的来源,TEa 值的几个测量值可能会出现变化:我们的研究旨在使用以下六种不同的 TEa 来源确定 20 个常规化学参数的西格玛分数:临床实验室改进修正案(CLIA 88′)、CLIA(临床实验室改进修正案)24、BDV(理想生物变异)、RCPA(澳大拉西亚皇家病理学家学院)、RiliBak(德国医学协会实验室医学检查质量保证指南)和 EMC/西班牙(Measurement of Laboratory Medical Examinations)、和 EMC/西班牙(测量和控制计划)在 12 个月内使用外部质量评估计划 (EQAS) 的偏差百分比和内部质量控制 (IQC) 的变异系数 (CV)。检测系统是自动、多通道、选择性分析仪 Beckman Coulter AU680,其工作原理是分光光度法。西格玛指标的计算公式为:西格玛 = (TEa - Bias%) / CV%。通过比较来自不同 TEa 来源的西格玛值,确定了 TEa 对西格玛指标评估的影响,然后针对表现不佳的参数制定了内部质量控制计划和 QGI(质量目标指数)。在 CLIA'88 中,TBil、HDL、CK、ALP、淀粉酶和尿酸是三个西格玛区以上的最大参数,而 RCPA 和生物变异被确定为最严重的参数,性能最高的参数低于三个西格玛区。Rilibaek 最宽松,只有钠和 CLIA'88 在三个西格玛区以下。研究结果表明,各种允许总误差(TEa)来源对西格玛指标评估有很大影响。使用 biorad unity 2.0 软件(westgard sigma multirules),根据分析物的不同西格玛分数制定了质量控制计划。该研究强调了统一和标准化西格玛指标的必要性,并强调了选择合适的总误差允许目标(TEa)的重要性。制定允许总误差(TEa)的全球标准和建议可促进其统一。要就各种实验室检测的可接受误差水平达成共识,需要许多国家和组织的专家合作,以制定此类准则和标准。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Beyond the basics: Sigma scores in laboratory medicine with variable total allowable errors (TEa)
Background
To diagnose diseases, track the effectiveness of treatments and make well-informed clinical decisions, doctors rely on results from laboratories. Accurate and precise results minimize the necessity for additional testing, saving time and money while enhancing patient satisfaction.. Internal quality control and an external quality assurance scheme(EQAS) are metrics used to evaluate a clinical laboratory’s performance. One of the numerous quality indicators that can be used to gauge the amount of errors is sigma metrics. To calculate the sigma scores bias%, CV%, and Total Error Allowable (TEa) are needed. Total Error allowable(TEa) is a crucial benchmark that establishes allowed limits on the degree of deviation from the target value for a certain analyte. Nevertheless, a proper consensus for establishing a TEa goal has not been reached and the impact of this limiting factor in standard laboratory practice and sigma calculation has not been sufficiently established. Choosing the right Total Error allowable(TEa) goal is one of the greatest challenges when employing sigma metrics as depending on the source, several measurands of TEa values may exhibit alteration.
Material and methods
Our study aims to determine the sigma scores of 20 routine chemistry parameters using six different TEa sources: Clinical Laboratory Improvement Amendment (CLIA 88′), CLIA(Clinical Laboratory Improvement Amendment) 24, BDV (Biological Variation Desirable), RCPA(Royal College of Pathologists of Australasia), RiliBak(Guideline of the German Medical Association for Quality Assurance of Laboratory Medical Examinations), and EMC/Spain(Measurement and Control Scheme) over a 12-month period using the bias percent from the External Quality Assessment Scheme (EQAS) and coefficient of variation (CV) from the Internal Quality Control (IQC). Detection system was automated, multi-channel, selective analyzer, the Beckman Coulter AU680 which works on the principle of spectrophotometry. To compute the Sigma metrics, formula used was Sigma = (TEa – Bias%) / CV%. By comparing the sigma values from the different TEa sources, TEa variance on the evaluation of the sigma metric was ascertained after which an internal quality control plan and QGI(Quality Goal Index) for underperforming parameters were devised.
Results
The study discovered that the sigma values of common chemical parameters varied significantly based on the TEa sources used. Maximum parameters in the above three-sigma zone were TBil, HDL, CK, ALP, amylase and uric acid in CLIA’88 while RCPA and Biological variation were determined to be the most severe, with the highest performing parameters falling below three sigma zones. Rilibaek was the most liberal, with only sodium in the lower three sigma zones along with CLIA’88. The findings indicate that there is the substantial influence of various Total Error Allowable (TEa) sources on the sigma metric evaluation. A quality control plan was devised depending on different sigma scores of the analytes using biorad unity 2.0 software(westgard sigma multirules). The origins of errors that resulted in low sigma ratings liked enhanced cleaning of electrodes, electrode replacement, ageing of reagents, instrument maintainence were pinpointed and addressed.
Conclusion
The study highlights the necessity of harmonizing and standardizing sigma metrics, stressing the significance of choosing suitable total error allowable goals (TEa). The creation of worldwide standards and recommendations for total error allowable (TEa) can lead to its harmonization. Establishing a consensus on the acceptable levels of error for various laboratory tests would necessitate the cooperation of specialists from many nations and organizations in order to set such guidelines and standards.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Clinica Chimica Acta
医学-医学实验技术
CiteScore
10.10
自引率
2.00%
发文量
1268
审稿时长
23 days
期刊介绍:
The Official Journal of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Clinica Chimica Acta is a high-quality journal which publishes original Research Communications in the field of clinical chemistry and laboratory medicine, defined as the diagnostic application of chemistry, biochemistry, immunochemistry, biochemical aspects of hematology, toxicology, and molecular biology to the study of human disease in body fluids and cells.
The objective of the journal is to publish novel information leading to a better understanding of biological mechanisms of human diseases, their prevention, diagnosis, and patient management. Reports of an applied clinical character are also welcome. Papers concerned with normal metabolic processes or with constituents of normal cells or body fluids, such as reports of experimental or clinical studies in animals, are only considered when they are clearly and directly relevant to human disease. Evaluation of commercial products have a low priority for publication, unless they are novel or represent a technological breakthrough. Studies dealing with effects of drugs and natural products and studies dealing with the redox status in various diseases are not within the journal''s scope. Development and evaluation of novel analytical methodologies where applicable to diagnostic clinical chemistry and laboratory medicine, including point-of-care testing, and topics on laboratory management and informatics will also be considered. Studies focused on emerging diagnostic technologies and (big) data analysis procedures including digitalization, mobile Health, and artificial Intelligence applied to Laboratory Medicine are also of interest.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


