通过 1 → 2 S 迁移合成 2-脱氧-2-氟-d-甘露糖结构单元并鉴定不常见的 2-S-苯基异构体吡啶三酸盐
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在使用 Selectfluor 与相应的糖醛类化合物时,2-脱氧-2-氟-d-甘露糖构件的区域和立体选择性合成路线往往具有实验挑战性。我们的目标是采用一种后期方法,通过三酯反转以立体特异的方式引入氟。因此,我们尝试用 C2-三酯与 TBAF 反转相应的 d-葡萄糖苷,合成了一种传统保护的 2-脱氧-2-氟-d-甘露糖 β-硫代糖苷供体,可直接用于寡糖合成。出乎意料的是,在吡啶中使用 Tf2O 形成 C2-三氟甲酸酯时,分离出了一种吡啶鎓盐。这可能是通过 1 → 2 S 迁移产生了 1,2-反式产物,该产物具有 α-d-manno 构型,而吡啶鎓的对映异构体处于假赤道位置。利用 X 射线晶体学技术确认了这一意想不到的固态中间体的结构。省略吡啶溶剂会导致二聚体的形成。将琼脂酮换成 O-对甲氧基苯基后,就能顺利地将 C2 反转为所需的 2-脱氧-2-氟 d-甘露糖体系,从而为进一步的同分异构体操作提供了适当的条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
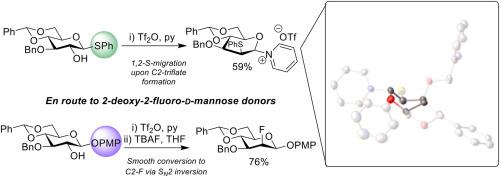
Towards the synthesis of a 2-deoxy-2-fluoro-d-mannose building block and characterisation of an unusual 2-S-phenyl anomeric pyridinium triflate salt via 1 → 2 S-migration
Regio- and stereo-selective synthetic routes to 2-deoxy-2-fluoro-d-mannose building blocks are often experimentally challenging when using Selectfluor with the corresponding glycal. We targeted a late-stage method to introduce fluorine in a stereospecific manner using inversion via a triflate. Accordingly, synthesis of a conventionally protected 2-deoxy-2-fluoro-d-mannose β-thioglycoside donor, directly applicable to oligosaccharide synthesis, was attempted using C2-triflate inversion of the corresponding d-glucoside with TBAF. Unexpectedly, an anomeric pyridinium salt was isolated when attempting to form the C2-triflate using Tf2O in pyridine. Indicatively, this proceeds via a 1 → 2 S-migration delivering a 1,2-trans product with α-d-manno configuration and the anomeric pyridinium in a pseudo-equatorial position. The structure of this unexpected intermediate was confirmed in the solid-state using X-ray crystallography. Omission of the pyridine solvent led to dimer formation. Switching the aglycone to an O-para-methoxyphenyl enabled smooth C2 inversion to the desired 2-deoxy-2-fluoro d-mannose system, suitably equipped for further anomeric manipulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


