铬氧化还原作用和动力学在 HxCrS2-δ 电化学循环中的作用
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
HxCrS2-δ 由 NaCrS2 的质子交换产生,具有晶体层和无定形层交替的特点。与母体化合物相比,HxCrS2-δ 具有更快的 Na+ 扩散速度、更高的容量和更好的循环性,因此作为钠离子电池电极表现出更优越的性能。这项研究利用粉末和单晶 X 射线衍射以及电子显微镜探索了 HxCrS2-δ 独特双相结构的性质。此外,还利用 X 射线吸收光谱、X 射线全散射和磁力测定法进行了原位表征,以研究这种优越性的产生机制。这些结果表明,铬的迁移并不会阻碍电池性能的提高,事实上,它可能是观察到的性能提高的关键。这些研究表明,在 HxCrS2-δ 中,铬的氧化还原不仅是可能的,而且含量很高,而在 NaCrS2 中以较低的电压进行氧化还原则会导致不可逆的结构转变,从而限制了循环稳定性。此外,我们还强调了 HxCrS2-δ 等双相结构在实现高性能储能电极方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
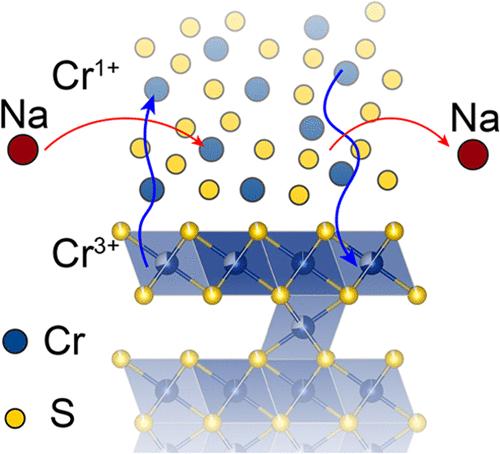
Role of Cr Redox and Dynamics in Electrochemical Cycling of HxCrS2−δ
HxCrS2−δ is produced by the proton exchange of NaCrS2 and features alternating layers of crystalline and amorphous lamella. It exhibits superior performance as a Na-ion battery electrode compared with its parent compound with faster Na+ diffusion, higher capacity, and better cyclability. This work explores the nature of the unique biphasic structure of HxCrS2−δ using both powder and single-crystal X-ray diffraction, as well as electron microscopy. Additionally, ex situ characterizations using X-ray absorption spectroscopy, X-ray total scattering, and magnetometry are employed to study the mechanism by which this superiority arises. These reveal that migration of Cr does not impede battery performance and may, in fact, be crucial to the observed performance improvements. These studies show that Cr redox is not only possible but abundant in HxCrS2−δ while accessing it in NaCrS2 at lower voltages results in irreversible structural transitions that limit cycling stability. Additionally, we highlight the potential of biphasic structures such as HxCrS2−δ to enable high performance in energy storage electrodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


