质子与电子:氧化还原不活跃的金属离子在高锰酸盐氧化动力学中的双重作用
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
氧化还原活性金属离子驱动的高价金属氧化络合物氧化行为调制在生物和化学合成中引起了极大的兴趣;然而,它们在高锰酸盐(Mn(VII))氧化去除有机污染物中的作用却在很大程度上被忽视了。在此,我们揭示了存在于水环境中的六种金属离子(即 Ca2+、Mg2+、Ni2+、Zn2+、Al3+ 和 Sc3+)对 Mn(VII) 活性的影响。电化学测试、硫代苯甲醚探针分析和动力学同位素效应都证明,这些离子在阻碍质子转移的同时,一致地增强了 Mn(VII) 的电子和氧转移能力。观察到的效应与金属离子的路易斯酸性密切相关。进一步的机理研究表明,锰(VII)可以与金属离子相互作用而不直接还原。这种相互作用改变了 Mn(VII)的电子构型,并创造了一个酸性微环境,从而增加了其亲电性和从有机底物中抽取质子的能量障碍。更重要的是,这些离子通过改变质子和电子转移的驱动力来调节锰(VII)去除酚类污染物的功效,即在 pH 值为 4.5 时促进质子和电子转移,而在较低的 pH 值时抑制质子和电子转移。此外,还讨论了活性锰中间体的贡献,以揭示金属离子/锰(VII)系统的氧化机制。这些发现不仅有助于合理设计金属离子存在时的锰(VII)氧化条件,以达到净化水的目的,还为增强亲电氧化作用提供了另一种范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
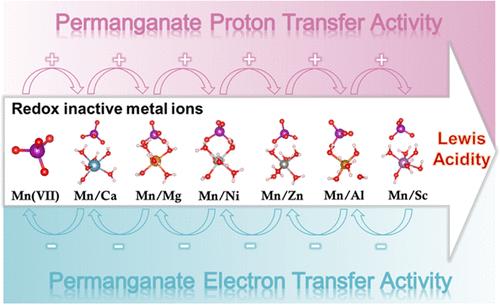
Proton vs Electron: The Dual Role of Redox-Inactive Metal Ions in Permanganate Oxidation Kinetics
Redox-inactive metal-ion-driven modulation of the oxidation behavior of high-valent metal–oxo complex has garnered significant interest in biological and chemical synthesis; however, their role in permanganate (Mn(VII)) oxidation for the removal of organic pollutants has been largely neglected. Here, we uncover the impact of six metal ions (i.e., Ca2+, Mg2+, Ni2+, Zn2+, Al3+, and Sc3+) presenting in water environments on Mn(VII) activity. These ions uniformly boost the electron and oxygen transfer capabilities of Mn(VII) while impeding proton transfer, as evidenced by electrochemical tests, thioanisole probe analysis, and the kinetic isotope effect. The observed effects are intricately linked to the Lewis acidity of the metal ions. Further mechanistic insights reveal that Mn(VII) can interact with metal ions without direct reduction. Such interactions modify the electronic configuration of Mn(VII) and create an acidic microenvironment, thus increasing its electrophilicity and the energy barrier for the abstraction of proton from organic substrates. More importantly, the efficacy of Mn(VII) in removing phenolic pollutants is regulated by these ions through changing the driving force for proton and electron transfer, i.e., facilitated at pH > 4.5 and inhibited at lower pH. The contribution of active Mn intermediates is also discussed to reveal the oxidative mechanism of the metal ion/Mn(VII) system. These findings not only facilitate the rational design of Mn(VII) oxidation conditions in the presence of metal ions for water decontamination but also offer an alternative paradigm for enhancing electrophilic oxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


