以选择性 PCK1 和 PGC-1α 赖氨酸乙酰化为靶点的小分子通过增加乳酸氧化作用发挥抗糖尿病作用
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
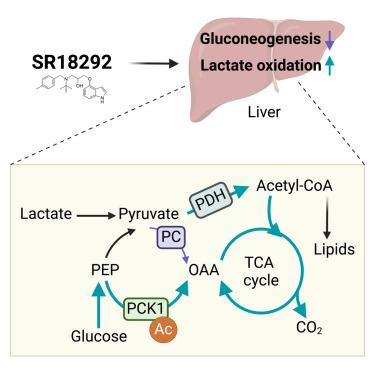
目前已经发现了一些小分子,它们能选择性地诱导过氧化物酶体增殖体激活受体-γ 辅激活剂(PGC)-1α 乙酰化,抑制胰高血糖素依赖性葡萄糖生成,从而产生抗糖尿病作用。然而,这些小分子如何选择性地抑制糖元代谢产物转化为葡萄糖而不干扰脂肪生成尚不清楚。在这里,我们发现小分子 SR18292 可通过增加乳酸和葡萄糖氧化来抑制肝糖生成。SR18292 可增加磷酸烯醇丙酮酸羧激酶 1(PCK1)的乙酰化,从而逆转其葡萄糖生成反应,有利于从磷酸烯醇丙酮酸合成草酰乙酸(OAA)。SR18292 诱导的 PCK1 反向催化反应为三羧酸(TCA)循环提供 OAA,是增加葡萄糖和乳酸氧化以及抑制葡萄糖生成所必需的。乙酰化模拟突变体 PCK1 K91Q 有利于无乙酰化反应,并能模拟 SR18292 在肝细胞中的代谢作用。肝脏特异性表达 PCK1 K91Q 突变体可改善肥胖小鼠的高血糖症状。因此,SR18292 通过 PCK1 赖氨酸乙酰化增强糖原底物氧化,从而阻断糖原生成,支持这些小分子药物的抗糖尿病作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Small molecules targeting selective PCK1 and PGC-1α lysine acetylation cause anti-diabetic action through increased lactate oxidation
Small molecules selectively inducing peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α acetylation and inhibiting glucagon-dependent gluconeogenesis causing anti-diabetic effects have been identified. However, how these small molecules selectively suppress the conversion of gluconeogenic metabolites into glucose without interfering with lipogenesis is unknown. Here, we show that a small molecule SR18292 inhibits hepatic glucose production by increasing lactate and glucose oxidation. SR18292 increases phosphoenolpyruvate carboxykinase 1 (PCK1) acetylation, which reverses its gluconeogenic reaction and favors oxaloacetate (OAA) synthesis from phosphoenolpyruvate. PCK1 reverse catalytic reaction induced by SR18292 supplies OAA to tricarboxylic acid (TCA) cycle and is required for increasing glucose and lactate oxidation and suppressing gluconeogenesis. Acetylation mimetic mutant PCK1 K91Q favors anaplerotic reaction and mimics the metabolic effects of SR18292 in hepatocytes. Liver-specific expression of PCK1 K91Q mutant ameliorates hyperglycemia in obese mice. Thus, SR18292 blocks gluconeogenesis by enhancing gluconeogenic substrate oxidation through PCK1 lysine acetylation, supporting the anti-diabetic effects of these small molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


