烯类对菲利普斯 Cr(VI) 催化剂的引发作用
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
为了更好地了解菲利普斯商用六价铬/二氧化硅催化剂的引发过程(即还原和自烷基化),本研究尝试对起始聚乙烯链上的引发基进行同位素标记。六价催化剂被氚化烯烃(C2D4 或 C3D6)还原,然后进行 C2H4 聚合。这在随后的聚合物 2H NMR 分析中放大了起始基团信号。在第二种方法中,六价铬催化剂被各种烯烃还原,然后通过注入原液进行水解。然后通过气相色谱-质谱分析氧化还原产物。这些数据表明,在 Cr(VI) 位点上形成的初始链以甲基开始。没有观察到不饱和现象。此外,在许多生成链中还发现了氧,这显然是在起始过程中从原始 Cr(VI) 中加入的。这表明还原和烷基化并不一定是单独和独立的反应,而往往是在一个协同过程中发生的。它进一步表明,起始机制并非只有一种,而是有多种,这取决于各个位点和所用单体的反应活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
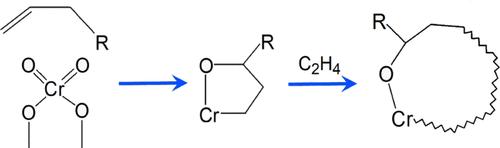
Initiation of the Phillips Cr(VI) Catalyst by Alkenes
As part of a continuing effort to better understand the initiation (i.e., the reduction and self-alkylation) of the Phillips commercial Cr(VI)/silica catalyst, this study attempted to isotopically label the initiating group on the starting PE chain. The hexavalent catalyst was reduced by deuterated olefin (C2D4 or C3D6), then polymerization of C2H4 was conducted. This amplified the starting group signal during the subsequent 2H NMR analysis of the polymer. In a second method, the Cr(VI) catalyst was reduced by various olefins, then hydrolyzed by injection of protic liquids. Redox products were then analyzed by GC–MS. These data indicate that the initial chain made on a Cr(VI) site begins with a methyl group. No unsaturation was observed. In addition, oxygen was found in many of the resultant chains, apparently incorporated from the original Cr(VI) as part of the initiation process. It demonstrates that reduction and alkylation are not necessarily separate and independent reactions, but often occur in a concerted process. It further suggests that there is not one initiation mechanism, but many, depending on the reactivity of the individual sites and the monomer used.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


