靶向纳米金簇通过非顺式铁凋亡协同治疗高风险神经母细胞瘤
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
颅外高危神经母细胞瘤(NB)患儿由于对细胞凋亡有抵抗力,预后较差。最近,在高危神经母细胞瘤的临床试验中测试了另一种细胞程序性死亡形式--铁凋亡,但也观察到了耐药性和副作用。在这里,我们发现金纳米团簇中的金元素能显著影响铁代谢,并使高危 NB 细胞对铁凋亡敏感。因此,我们开发了一种与改良的 NB 靶向肽共轭的金纳米簇。这种金纳米簇(即 NANT)显示出卓越的 NB 靶向效率,并能显著促进铁跃迁。令人惊讶的是,这种效应是通过提升非规范的铁突变途径产生的,而这种途径依赖于血红素加氧酶-1调控的铁(II)积累。此外,NANT 通过促进非典型铁突变,显著抑制了高危 NB 在肿瘤小球和异种移植模型中的生长,瘤内铁(II)和血红素加氧酶-1 的增强证明了这一点。重要的是,这种策略显示出极佳的心脏安全性,为克服铁蛋白沉积抗性、高效安全地治疗高危神经母细胞瘤患儿提供了一种前景广阔的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
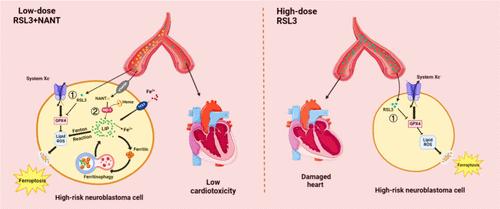
Targeted Gold Nanoclusters for Synergistic High-Risk Neuroblastoma Therapy through Noncanonical Ferroptosis
Children with extracranial high-risk neuroblastoma (NB) have a poor prognosis due to resistance against apoptosis. Recently, ferroptosis, another form of programmed cell death, has been tested in clinical trials for high-risk NB; however, drug resistance and side effects have also been observed. Here, we find that the gold element in gold nanoclusters can significantly affect iron metabolism and sensitize high-risk NB cells to ferroptosis. Accordingly, we developed a gold nanocluster conjugated with a modified NB-targeting peptide. This gold nanocluster, namely, NANT, shows excellent NB targeting efficiency and dramatically promotes ferroptosis. Surprisingly, this effect is exerted by elevating the noncanonical ferroptosis pathway, which is dependent on heme oxygenase-1-regulated Fe(II) accumulation. Furthermore, NANT dramatically inhibits the growth of high-risk NB in both tumor spheroid and xenograft models by promoting noncanonical ferroptosis evidenced by enhanced intratumoral Fe(II) and heme oxygenase-1. Importantly, this strategy shows excellent cardiosafety, offering a promising strategy to overcome ferroptosis resistance for the efficient and safe treatment of children with high-risk neuroblastoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


