重新审视有序反萤石型 Li14Cr2N8O:合成、晶体结构、理论视角和氨分解催化活性
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
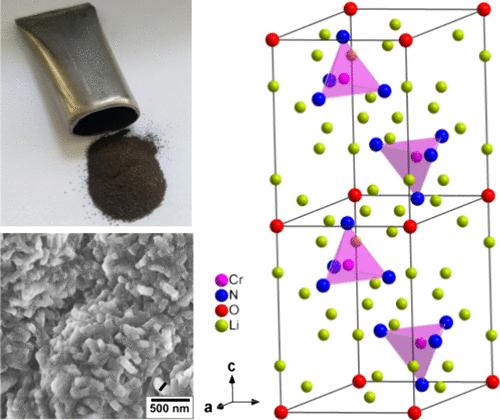
氨是一种高效的氢气运输化合物。使用市售催化剂对氨进行催化分解,可在使用点将氢从氨中释放出来。过渡金属氮化物具有与著名的贵金属基催化剂相似的特性,在氨分解中表现出卓越的催化活性。特别是某些锂基三元金属氮化物的循环形成和分解,提高了氨分解的催化活性。由于金属氮化锂氧化物的稳定性通常超过金属氮化锂,因此基于金属氮化锂氧化物的催化剂在未来的实际应用中具有特别重要的意义。因此,本研究旨在从理论角度重新审视锂基过渡金属氮氧化物家族成员 Li14Cr2N8O 的合成、晶体结构和稳定性。研究采用一步法成功合成了高结晶度的单相粉末状 Li14Cr2N8O。确定了 Li14Cr2N8O 的晶体结构为 Na14Mn2O9 型结构。应用贝尼豪森形式主义阐明了反萤石型结构与具有 Na14Mn2O9 型结构的 Li14Cr2N8O 之间的基团-亚基关系。里特维尔德精炼法和文献中的原子参数显示出同样令人满意的结果。Li14Cr2N8O 的单位晶胞参数为 a = 5.7936(6) Å 和 c = 8.20634(11) Å(三方晶系)。通过密度泛函理论(DFT)进行理论研究,探讨了 Li14Cr2N8O 在阴离子顺序和交换、磁基态以及铬的氧化态方面的稳定性。本研究的发现为进一步探索 Li14Cr2N8O 作为分解氨生成氢气的重要催化剂铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Revisiting Ordered Antifluorite-Type Li14Cr2N8O: Synthesis, Crystal Structure, Theoretical Perspectives, and Catalytic Activity for Ammonia Decomposition
Ammonia is an efficient compound for hydrogen transportation. The release of hydrogen from ammonia at the point of use is accomplished by the catalytic decomposition of ammonia using commercially available catalysts. Transition metal nitrides with properties similar to those of well-known precious metal-based catalysts exhibit outstanding catalytic activity in ammonia decomposition. Particularly, the cyclical formation and decomposition of certain lithium-based ternary metal nitrides result in improved catalytic activity in the decomposition of ammonia. Since the stability of lithium metal nitride oxides generally exceeds that of lithium metal nitrides, catalysts based on lithium metal nitride oxides are of particular interest for future practical applications. Therefore, this study aims at revisiting the synthesis, crystal structure, and stability from the theoretical perspective of Li14Cr2N8O, as a member of the lithium-based transition metal nitride oxide family. A one-step method is applied to successfully synthesize single-phase Li14Cr2N8O in powder form with high crystallinity. The crystal structure of Li14Cr2N8O is determined to adopt the Na14Mn2O9-type structure. The group-subgroup relation between the antifluorite-type structure and Li14Cr2N8O with the Na14Mn2O9-type structure is elucidated by applying the Bärnighausen formalism. Rietveld refinements and atomic parameters from the literature show equally satisfactory results. The unit-cell parameters obtained for Li14Cr2N8O are a = 5.7936(6) Å and c = 8.20634(11) Å (trigonal crystal system). Theoretical studies by density functional theory (DFT) are conducted to explore the stability of Li14Cr2N8O with respect to anion order and exchange, the magnetic ground state, and the oxidation state of chromium. The findings of this study pave the way for Li14Cr2N8O to be further explored as an important catalyst for the decomposition of ammonia to generate hydrogen.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


