生物催化不对称醛醇加成到未活化酮中
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
酶以其催化效率和选择性而闻名,但有机合成中的许多经典转化却没有相应的生物催化剂。醛醇酶是一种神奇的 C-C 键形成酶,但只有在特殊情况下,它们的反应活性才会超过活化的羰基亲电物。为了探究这种限制的机理根源,我们使用了一对活性依赖于磷酸吡哆醛的醛缩酶。我们的研究结果揭示了醛缩酶是如何受限于与溶剂之间在动力学上有利的质子转移,从而破坏了酮的醛加成作用。我们展示了一种反醛醇酶如何绕过这一限制,实现与未活化酮的高效加成。由此产生的产物是非常受欢迎的非典型氨基酸,其侧链含有手性叔醇。机理分析表明,反醛酶活性是磷酸吡哆醛化学的一个固有特征,并确定了扩展醛酶催化作用的原理,使其超越以往的限制,从而能够从简单的起始材料中形成会聚的、对映选择性的 C-C 键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
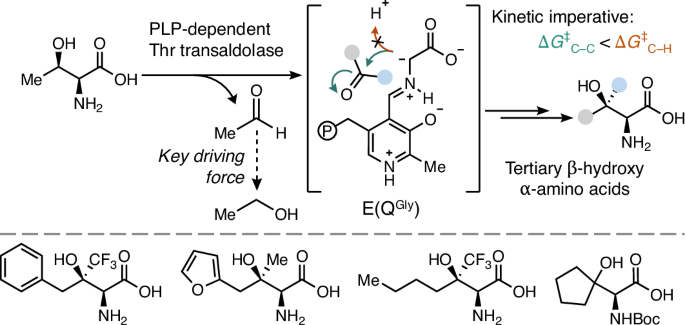
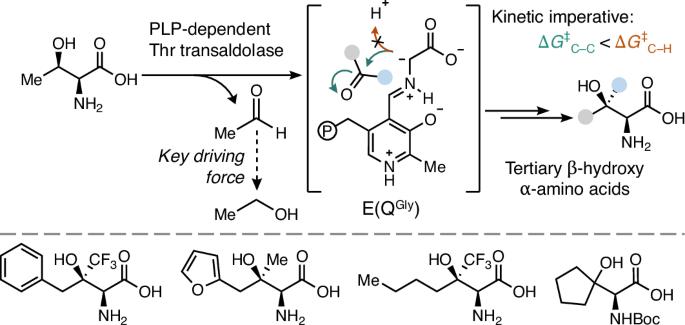
Biocatalytic asymmetric aldol addition into unactivated ketones
Enzymes are renowned for their catalytic efficiency and selectivity, but many classical transformations in organic synthesis have no biocatalytic counterpart. Aldolases are prodigious C–C bond-forming enzymes, but their reactivity has only been extended past activated carbonyl electrophiles in special cases. To probe the mechanistic origins of this limitation, we use a pair of aldolases whose activity is dependent on pyridoxal phosphate. Our results reveal how aldolases are limited by kinetically favourable proton transfer with solvent, which undermines aldol addition into ketones. We show how a transaldolase can circumvent this limitation, enabling efficient addition into unactivated ketones. The resulting products are highly sought non-canonical amino acids with side chains that contain chiral tertiary alcohols. Mechanistic analysis reveals that transaldolase activity is an intrinsic feature of pyridoxal phosphate chemistry and identifies principles for extending aldolase catalysis beyond its previous limits to enable convergent, enantioselective C–C bond formation from simple starting materials. Aldolases have been a mainstay in synthesis, but their scope has been limited to activated electrophiles. Now carbon–carbon bond formation with ketone electrophiles is enabled by transaldolases, which form a strong nucleophile that is resistant to protonation. This chemistry enables convergent synthesis of non-canonical amino acids bearing tertiary alcohol side chains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


