功能化三维打印凝胶MA/皂石水凝胶支架通过AMPK/mTOR信号通路促进骨免疫调节增强成骨作用,从而促进BMSCs募集
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨髓间充质干细胞(BMSCs)的迁移和分化在骨修复过程中起着至关重要的作用。然而,传统的支架往往不能有效诱导和招募骨髓间充质干细胞。在我们的研究中,我们提出了一种新方法,即引入一种由水凝胶组成的三维生物打印支架,并在 GelMA 溶液中添加青石,旨在提高支架的性能。体内和体外实验都证实了该支架出色的生物相容性。此外,Apt19s 首次被化学修饰到水凝胶支架表面,从而显著增强了 BMSCs 的迁移和粘附能力。此外,该支架在体内和体外环境中都表现出了强大的成骨分化能力。此外,水凝胶支架还能诱导巨噬细胞从 M1 极化为 M2,从而通过骨免疫途径促进 BMSCs 的成骨分化。通过 RNA-seq 分析发现,巨噬细胞通过 AMPK/mTOR 信号通路调控 BMSCs 的成骨分化。总之,功能化 GelMA/Laponite 支架为定制原位骨再生提供了一种经济有效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
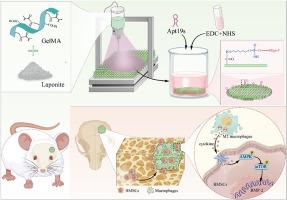
Functionalized 3D-printed GelMA/Laponite hydrogel scaffold promotes BMSCs recruitment through osteoimmunomodulatory enhance osteogenic via AMPK/mTOR signaling pathway
The migration and differentiation of bone marrow mesenchymal stem cells (BMSCs) play crucial roles in bone repair processes. However, conventional scaffolds often lack of effectively inducing and recruiting BMSCs. In our study, we present a novel approach by introducing a 3D-bioprinted scaffold composed of hydrogels, with the addition of laponite to the GelMA solution, aimed at enhancing scaffold performance. Both in vivo and in vitro experiments have confirmed the outstanding biocompatibility of the scaffold. Furthermore, for the first time, Apt19s has been chemically modified onto the surface of the hydrogel scaffold, resulting in a remarkable enhancement in the migration and adhesion of BMSCs. Moreover, the scaffold has demonstrated robust osteogenic differentiation capability in both in vivo and in vitro environments. Additionally, the hydrogel scaffold has shown the ability to induce the polarization of macrophages from M1 to M2, thereby facilitating the osteogenic differentiation of BMSCs via the bone immune pathway. Through RNA-seq analysis, it has been revealed that macrophages regulate the osteogenic differentiation of BMSCs through the AMPK/mTOR signaling pathway. In summary, the functionalized GelMA/Laponite scaffold offers a cost-effective approach for tailored in situ bone regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


