模型 Rh/Fe3O4(001) 催化剂在氢环境中的演变
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
分散在氧化物上的单金属原子因其独特的电子和化学特性而成为一类新兴催化剂。在本研究中,我们在 Fe3O4(001) 上制备了一系列具有特征良好的 Rh 位点的模型单原子催化剂,这些位点包括 Rh adatoms (Rhad)、八面体配位的 Rh 混合表层 (Rhoct),以及金属 Rh 簇和纳米颗粒 (Rhmet)。我们利用 X 射线光电子能谱 (XPS) 和扫描隧道显微镜 (STM) 研究了这些模型系统对 H2 的活性及其在还原环境中的稳定性。结果表明,原子分散的 Rhad 和 Rhoct 物种不会激活 H2,从而导致在 Fe3O4(001) 上形成表面羟基。相反,Rhmet 在 H2 中的存在会导致羟基的形成,随后在较高温度下(≥ 500 K),由于通过 Mars-van Krevelen 机制形成的水,Fe3O4(001)会被蚀刻。此外,这种表面蚀刻会导致 Rhoct 从表面晶格中释放出来,并烧结成 Rhmet。为了缩小表面科学模型与高比表面积催化剂之间的材料差距,我们对 Rh/Fe3O4 粉末催化剂进行了平行研究。XPS 表征显示了这些体系之间的显著相似性。此外,我们的表面科学研究还提供了高比表面积催化剂在 H2 大气中行为的原子图景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
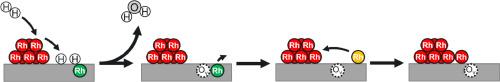
The evolution of model Rh/Fe3O4(001) catalysts in hydrogen environments
Single metal atoms dispersed on oxides are a new emerging class of catalysts owing to their unique electronic and chemical properties. In this study, we have prepared a series of model single-atom catalysts possessing well-characterized Rh sites that include Rh adatoms (Rhad), mixed surface layers with octahedrally-coordinated Rh (Rhoct), as well as metallic Rh clusters and nanoparticles (Rhmet) on Fe3O4(001). Using X-ray photoelectron spectroscopy (XPS) and scanning tunneling microscopy (STM), we investigated the activity of such model systems towards H2 and their stability in reducing environments. Our results show that the atomically dispersed Rhad and Rhoct species do not activate H2, which would result in the formation of surface hydroxyls on Fe3O4(001). In contrast, the presence of Rhmet in H2 results in the formation of hydroxyls and subsequent etching of the Fe3O4(001) at higher temperatures (≥ 500 K) due to water formation via the Mars−van Krevelen mechanism. Additionally, such surface etching leads to the release of the Rhoct from the surface lattice and their sintering to Rhmet. To bridge the material gap between the surface science models and high surface area catalysts, we perform parallel studies on powder Rh/Fe3O4 catalysts. The XPS characterization shows remarkable similarities between these systems. Further, our surface science studies provide an atomistic picture of the behavior of high surface area catalysts in the H2 atmosphere.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


