组蛋白 H3 赖氨酸 27 上的三甲基化(H3K27me3)在脑胶质瘤的替莫唑胺耐药性中的作用
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
替莫唑胺(TMZ)是治疗恶性胶质瘤的一线化疗药物,但其耐药性限制了患者的治疗效果。本研究探讨了组蛋白H3赖氨酸27的三甲基化(H3K27me3)在TMZ耐药性中的作用和意义。研究收集了20名晚期胶质瘤患者的数据,并对其病理样本的H3K27me3水平进行了分析。比较了胶质瘤细胞U87和TMZ耐药细胞U87TR对TMZ的敏感性,并测定了两种细胞的H3K27me3水平。评估了H3K27me3去甲基化酶抑制剂GSK-J4与TMZ联合使用对U87TR细胞增殖和迁移的影响。结果表明,高水平的H3K27me3可预测接受TMZ治疗的胶质瘤患者更长的无病生存期(DFS)和总生存期(OS)。与U87细胞相比,U87TR细胞的H3K27me3水平较低。GSK-J4提高了U87TR细胞的H3K27me3水平,降低了它们对TMZ的耐药性。总之,本研究发现了胶质瘤中TMZ耐药性的新标记物,并提供了应对这一挑战的新策略。这些发现对未来改善胶质瘤的临床治疗具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
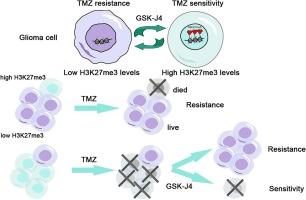
The role of trimethylation on histone H3 lysine 27 (H3K27me3) in temozolomide resistance of glioma
Temozolomide (TMZ) is the first-line chemotherapeutic agent for malignant glioma, but its resistance limited the benefits of the treated patients. In this study, the role and significance of trimethylation of histone H3 lysine 27 (H3K27me3) in TMZ resistance were investigated. Data from twenty advanced glioma patients were collected, and their pathological samples were analyzed for H3K27me3 levels. TMZ sensitivity was compared between glioma cells U87 and TMZ-resistant cells U87TR, with H3K27me3 levels determined in both cells. The effects of H3K27me3 demethylases inhibitor GSK-J4, combined with TMZ, were assessed on the proliferation and migration of U87TR cells. The results indicated that a high level of H3K27me3 predicts longer disease free survival (DFS) and overall survival (OS) in glioma patients receiving TMZ treatment. The H3K27me3 level was lower in U87TR cells compared to U87 cells. GSK-J4 increased the H3K27me3 level in U87TR cells and decreased their resistance to TMZ. In summary, this study identified a novel marker of TMZ resistance in glioma and provided a new strategy to address this challenge. These findings are significant for improving the clinical treatment of glioma in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


