N-取代的 4-苯基-2-氨基噻唑衍生物的合成及其对 hCA I、II 和 AChE 酶抑制特性的研究
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究合成了含有磺酰胺、酰胺和苯基氨基的噻唑衍生物,以保护 5-甲基-4-苯基-2-氨基噻唑和 4-苯基-2-氨基噻唑的游离氨基。研究了 N 保护噻唑衍生物的卤化反应。利用 LCMS、FT-IR、1H NMR 和 13C NMR 光谱技术阐明了合成化合物的结构。研究了 N 保护噻唑衍生物对人碳酸酐酶 I、II(hCA I、hCA II)和乙酰胆碱酯酶(AChE)的抑制作用。合成的 N-保护噻唑衍生物中效果最好的对 hCA I 的 Ki 值范围为 46.85-587.53 nM,对 hCA II 的 Ki 值范围为 35.01-578.06 nM,对 AChE 的 Ki 值范围为 19.58-226.18 nM。此外,通过对实验结果最好的噻唑衍生物(9 和 11)的目标酶进行硅学研究,考察了相关化合物在酶活性位点的结合相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
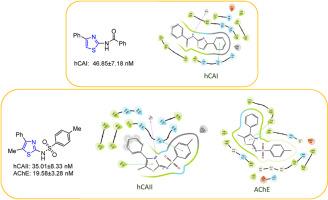
Synthesis of N-substituted 4-phenyl-2-aminothiazole derivatives and investigation of their inhibition properties against hCA I, II, and AChE enzymes
In this study, thiazole derivatives containing sulphonamide, amide, and phenyl amino groups were synthesized to protect the free amino groups of 5-methyl-4-phenyl-2-aminothiazole and 4-phenyl-2-aminothiazole. Halogenated reactions of N-protected thiazole derivatives have been investigated. LCMS, FT-IR, 1H NMR, and 13C NMR spectroscopy techniques were used to elucidate the structures of the synthesized compounds. Inhibition effects of the N-protected thiazole derivatives against human carbonic anhydrase I, II (hCA I, hCA II), and acetylcholinesterase (AChE) were investigated. The best results among the synthesized N-protected thiazole derivatives showed Ki values in the range of 46.85–587.53 nM against hCA I, 35.01–578.06 nM against hCA II, and in the range of 19.58–226.18 nM against AChE. Furthermore, in silico studies with the target enzyme of the thiazole derivatives (9 and 11), which showed the best results experimentally, have examined the binding interactions of the related compounds at the enzyme active site.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


