线粒体基因组衍生的 circRNA:分子生物学中的 "孤儿 "表观遗传调节因子
IF 4.5
3区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
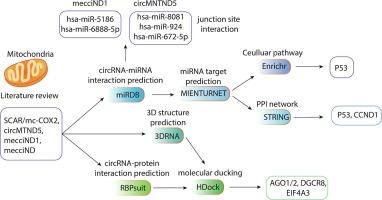
线粒体对细胞活动至关重要,影响着 ATP 生成、Ca2+ 信号传导和活性氧生成。有人提出,核基因组衍生的环状 RNA(circRNA)在生物过程中发挥作用。本研究首次旨在通过内嵌分析,全面探索经实验证实的人类线粒体基因组衍生的环状核糖核酸(mt-circRNAs)。我们利用广泛的生物信息学工具来预测它们在分子生物学中的作用,包括 miRNA 海绵作用、蛋白质拮抗作用和肽翻译作用。在五种表征明确的mt-circRNA中,SCAR/mc-COX2尤为突出,它具有海绵状作用的潜力,可吸附约41种不同的miRNA,这些miRNA主要靶向参与内吞、MAP激酶和PI3K-Akt通路的多个基因。有趣的是,circMNTND5 和 mecciND1 通过其独特的后接合序列与 miRNA 发生特异性相互作用。这些专门靶向的 miRNA(has-miR-5186、6888-5p、8081、924、672-5p)主要与胰岛素分泌、癌症中的蛋白多糖和 MAPK 信号通路有关。此外,所有 mt-circRNA 都会通过 miRNA 封存作用对 P53 通路产生错综复杂的影响。值得注意的是,mc-COX2 和 circMNTND5 似乎通过拮抗 AGO1/2、EIF4A3 和 DGCR8 参与了 RNA 的生物发生。所有 mt-circRNA 都与氧化还原信号转导中至关重要的 IGF2BP 蛋白相互作用,除 mecciND1 外,它们都可能生成至少一种类似免疫球蛋白重链蛋白的蛋白质。鉴于 P53 具有氧化还原敏感转录因子的功能,而胰岛素是能量代谢的关键调节因子,它们与 mt-circRNA 的间接相互作用可能会影响细胞的结果。然而,由于关注度有限和数据不经常获得,最好进行更深入的研究,以更深入地了解 mt-circRNA 的功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mitochondrial genome-derived circRNAs: Orphan epigenetic regulators in molecular biology
Mitochondria are vital for cellular activities, influencing ATP production, Ca2+ signaling, and reactive oxygen species generation. It has been proposed that nuclear genome-derived circular RNAs (circRNAs) play a role in biological processes. For the first time, this study aims to comprehensively explore experimentally confirmed human mitochondrial genome-derived circRNAs (mt-circRNAs) via in-silico analysis. We utilized wide-ranging bioinformatics tools to anticipate their roles in molecular biology, involving miRNA sponging, protein antagonism, and peptide translation. Among five well-characterized mt-circRNAs, SCAR/mc-COX2 stands out as particularly significant with the potential to sponge around 41 different miRNAs, which target several genes mostly involved in endocytosis, MAP kinase, and PI3K-Akt pathways. Interestingly, circMNTND5 and mecciND1 specifically interact with miRNAs through their unique back-splice junction sequence. These exclusively targeted miRNAs (has-miR-5186, 6888-5p, 8081, 924, 672-5p) are predominantly associated with insulin secretion, proteoglycans in cancer, and MAPK signaling pathways. Moreover, all mt-circRNAs intricately affect the P53 pathway through miRNA sequestration. Remarkably, mc-COX2 and circMNTND5 appear to be involved in the RNA’s biogenesis by antagonizing AGO1/2, EIF4A3, and DGCR8. All mt-circRNAs engaged with IGF2BP proteins crucial in redox signaling, and except mecciND1, they all potentially generate at least one protein resembling the immunoglobulin heavy chain protein. Given P53′s function as a redox-sensitive transcription factor, and insulin’s role as a crucial regulator of energy metabolism, their indirect interplay with mt-circRNAs could influence cellular outcomes. However, due to limited attention and infrequent data availability, it is advisable to conduct more thorough investigations to gain a deeper understanding of the functions of mt-circRNA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mitochondrion
生物-细胞生物学
CiteScore
9.40
自引率
4.50%
发文量
86
审稿时长
13.6 weeks
期刊介绍:
Mitochondrion is a definitive, high profile, peer-reviewed international research journal. The scope of Mitochondrion is broad, reporting on basic science of mitochondria from all organisms and from basic research to pathology and clinical aspects of mitochondrial diseases. The journal welcomes original contributions from investigators working in diverse sub-disciplines such as evolution, biophysics, biochemistry, molecular and cell biology, genetics, pharmacology, toxicology, forensic science, programmed cell death, aging, cancer and clinical features of mitochondrial diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


