从电动汽车废锂离子电池黑块产生的草酸浸出液中回收锂
IF 5.5
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
由于锂离子电池(LIB)在电动汽车(EV)中的应用,锂(Li)的需求急剧增加。为了减少原始资源的供应,并满足新的立法回收目标,必须确保二次资源的供应。最近有报道称,草酸浸出法可用于从废旧电动汽车锂离子电池(LIB)的黑块(BM)中进行早期锂回收。此类工艺通常在电极分离、阴极材料热处理和浸出液浓缩方面面临挑战。在此,我们首次展示了苯甲酰基三氟丙酮(HBTA)和三辛基氧化膦(TOPO)的协同应用,以从工业 BM 的草酸盐流中提取锂。在最佳条件下,水相/有机相(A/O)比例为 1,pH 值为 11,6 分钟内就能从浸出液中提取出 ∼92 % 的锂。McCabe Thiele 图显示,在水相/有机相比率为 1 时,有两个逆流阶段可以完全萃取锂。斜率分析法表明萃取锂所需的 HBTA 的摩尔数相等,傅立叶变换红外光谱证实了萃取剂的协同作用。有机相用锂盐洗涤,并用盐酸(HCl)剥离,得到浓缩(锂 ∼ 4 mol/L)溶液,沉淀为碳酸锂(纯度为 99%)。在草酸浸出过程中,过渡金属因溶解度低而被还原并沉淀为相应的草酸盐。使用盐酸进行第二步浸出后,过渡金属被成功回收。过量的酸通过冷却结晶以草酸形式回收,这是早先未报道过的 BM 回收流,从而促进了闭环工艺。所开发的流程大大推进了从 BM 中回收锂的早期工艺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
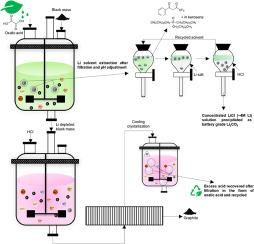
Recovery of lithium from oxalic acid leachate produced from black mass of spent electric vehicle Li-ion batteries
Lithium (Li) demand is surging due to the adaptation of Lithium-ion batteries (LIBs) in electric vehicles (EV). To ease the supply from virgin sources and meet new legislative recycling targets it's imperative to secure supply from secondary sources. Recently oxalic acid leaching has been reported for early-stage recovery of Li from black mass (BM) of spent EV LIBs. Such processes often face challenges in electrodes separation, thermal treatment of cathode material, and leachate up-concentration. Herein, the synergistic application of benzoyltrifluoroacetone (HBTA) and trioctylphosphine oxide (TOPO) to extract Li from oxalate streams of an industrial BM has been demonstrated for the first time. Under optimum conditions, ∼92 % Li was extracted from the leachate in 6mins, aqueous/organic (A/O) phase ratio 1, and pH 11. McCabe Thiele diagram indicated two counter-current stages for the complete extraction of Li at A/O 1. The slope analysis method indicated equal moles of HBTA required to extract Li and FT-IR spectroscopy confirmed the synergism of the extractants. The organic phase was scrubbed with Li salt and stripped with hydrochloric acid (HCl) resulting in a concentrated (Li ∼4 mol/L) solution precipitated as lithium carbonate (>99 % pure). The transition metals during oxalic acid leaching were reduced and precipitated as respective oxalates due to low solubility. They were successfully recovered using a second leaching step with HCl. The excess acid was recovered as oxalic acid by cooling crystallization, not reported earlier for BM recycling streams, facilitating a closed-loop process. The developed flowsheet significantly advances the early-stage Li recovery processes from BM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


