在黑色 TiO2 上通过电催化还原草酸绿色合成乙醇酸:实验和理论研究
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
在此,我们介绍了以黑色 TiO2 为电催化剂将草酸氢化成乙醇酸的电催化四电子氢化过程。草酸是一种存在于多种有机废物中的丰富化合物。研究结果表明,黑色二氧化钛对乙醇酸具有高选择性,乙醛酸的形成是限速步骤(乙醛酸是双电子中间体)。使用室温下运行的 H 型电池,在 10.2 mA cm-2 的条件下电解 4 小时,法拉第效率(FE)最高,为 69.6% ± 8.3%,草酸转化率为 50.2% ± 3.8%(降解动力学常数 k = 0.0042 ± 0.0001 min-1),反应产率为 58.8% ± 7.0%,乙醇酸产量为 1.2 ± 0.18 g L-1。通过引入 Ti3+ 缺陷,实现了由锐钛矿型二氧化钛衍生出黑色二氧化钛的理论模型,从而使黑色二氧化钛理论上能够像实验观察到的那样轻松地将草酸转化为乙醇酸。密度泛函理论计算支持并详细描述了反应机理,发现表面 Ti3+ 状态是主要催化位点。这是首次在原子水平上为这一电催化反应提出详细的分步机理,对理解这一具有高能量/环境意义的过程做出了宝贵贡献。这也是黑色二氧化钛首次被用作这一可持续过程的电催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
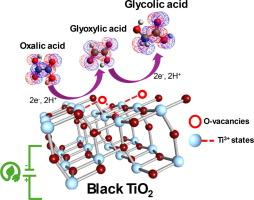
Green synthesis of glycolic acid through the electrocatalytic reduction of oxalic acid over black TiO2: An experimental and theoretical study
Herein, we present the electrocatalytic four-electron hydrogenation of oxalic acid into glycolic acid using black TiO2 as an electrocatalyst. Oxalic acid is an abundant compound found in several sources of organic waste. The results showed a high selectivity of black TiO2 toward glycolic acid, with the formation of glyoxylic acid being the rate-limiting step (glyoxylic acid is the two-electron intermediate). The highest Faradaic efficiency (FE) of 69.6% ± 8.3% was achieved at 10.2 mA cm−2 in 4 h of electrolysis using an H-type cell operated at room temperature, with 50.2% ± 3.8% of oxalic acid conversion (degradation kinetic constant k = 0.0042 ± 0.0001 min−1), 58.8% ± 7.0% of reaction yield and 1.2 ± 0.18 g L−1 of glycolic acid production. A theoretical model of black TiO2 coming from anatase TiO2 was implemented by introducing Ti3+ defects, which gave black TiO2 the theoretical capability to easily transform oxalic acid into glycolic acid as experimentally observed. The reaction mechanism was supported and described in detail by density functional theory calculations, which revealed that surface Ti3+ states were the main catalytic sites. This is the first time that a detailed step-by-step mechanism at the atomic level has been proposed for this electrocatalytic reaction, which represents a valuable contribution to the understanding of this process of high energy/environmental interest. This is also the first time that black TiO2 has been used as an electrocatalyst for this sustainable process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


