探索坎普三酸衍生物感知 G 系列神经毒剂的反应机理途径:计算研究
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
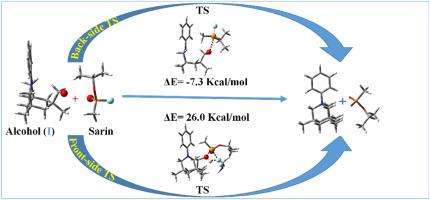
据报道,坎普三酸衍生物是利用光诱导电子转移(PET)过程感测神经毒剂的候选物质。在制备传感器探针分子时,伯醇非常靠近叔胺,使伯醇酰化,并通过快速的分子内 N-烷基化反应生成季铵盐。季铵盐和丙基膦酸异甲酯的生成反应途径仍未探明。本报告通过计算探索了 G 系列神经毒剂沙林及其模拟物二乙基胆磷酸酯(DCP)与坎普三酸衍生物的机理途径。在 DCM 溶剂中用 B3LYP-D3/6-311+G(d,p) 理论水平进行的计算表明,反应是通过分子间和分子内 SN2 途径进行的。探针分子受到立体阻碍,因此研究了前侧和后侧 SN2 反应途径。计算结果表明,坎普三酸衍生物(I & II)与沙林的第一次分子间 SN2 反应的背面攻击在能量上比正面攻击有利,而接下来的分子内 SN2 反应则是一个无障碍过程。用模拟物二乙基胆磷酸酯(DCP)和坎普三酸衍生物(I & II)进行的计算表明,与神经毒剂沙林分子相比,这些反应在能量上更为容易。畸变-相互作用模型和ΔNBOSteric分析表明,在此类SN2反应中,背面攻击在能量上比正面攻击更有利。设计出的带有磷中心的 Kemp's 三酸衍生物 (II) 可感知沙林和 (DCP),这表明杂中心的大小对于促进探针分子中的反应非常重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the reaction mechanistic pathway for the sensing of G-Series nerve agent with Kemp's triacid derivative: A computational study
Kemp's triacid derivative have been reported as a candidate for sensing of nerve agents employing the photoinduced electron transfer (PET) processes. The sensor probe molecules were prepared with primary alcohol in very close proximity to a tertiary amine to acylate the alcohol with rapid intramolecular N-alkylation to produce quaternary ammonium salt. The reaction pathways for the formation of quaternary ammonium salt and isomethyl propyl phosphonate remain unexplored. In this report, the mechanistic pathways of G-series nerve agents sarin and its simulant diethylcholorophosphate (DCP) with Kemp's triacid derivatives has been explored computationally. The calculations performed with B3LYP-D3/6-311+G(d,p) level of theory in DCM solvent revealed that the reaction proceeds via intermolecular and intramolecular SN2 pathways. The probe molecule is sterically hindered, therefore, the frontside and backside SN2 reaction pathways have been examined. The computed results suggest that the first intermolecular SN2 reaction of Kemp's triacid derivatives (I & II) with sarin for the backside attack is energetically favored compared to the frontside attack and the following intramolecular SN2 reaction is a barrierless process. The calculations performed with simulant diethylcholorophosphate (DCP) and Kemp's triacid derivatives (I & II) show that the reactions are energetically more facile compared to the nerve agent sarin molecule. The Distortion-Interaction model and ΔNBOSteric analysis showed that the back side is energetically favored over the front side attack in such SN2 reactions. The designed Kemp's triacid derivative (II) with phosphorus center for sensing sarin and (DCP) suggests that the size of the hetero centers are important to facilitate the reaction in the probe molecule.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


