利用物理球接触模型研究平面六边形、方形和三棱晶格上支撑的紧密堆积纳米粒子的结构
IF 2
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
随着异相催化和材料科学的发展,纳米粒子的定制设计变得越来越重要。为了提高催化活性和选择性,有必要在催化剂中形成具有活性位点特定几何形状的纳米粒子。在此,我们使用物理球接触模型,在平面紧密堆积表面上建立了六角形、方形和三 角形几何形状的各种纳米粒子,以了解在纳米粒子支撑金属的简化模型中,(100) 和 (111) 位点的分布如何随纳米粒子(NP)尺寸的变化而变化。这种方法的结果清楚地表明,在具有六角形基底的 2 层 NP 中,与 NP 中的原子数相比,其 (100) 位点的数量是方形和三 角形基底 NP 的 2-3 倍。在三维各向同性 NP 中,这种现象比两层 NP 更为明显。我们推导出了估算 (100)、(111)、原子数和长宽比与 n 的函数关系的方程。这些方程对于调整支撑在紧密堆积金属表面上的 NPs 的性质非常重要,这些 NPs 可应用于材料科学、纳米技术和催化等领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
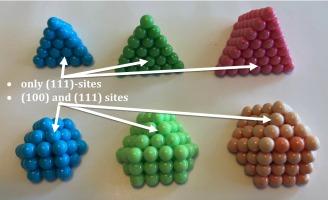
A study using physical sphere-in-contact models to investigate the structure of close-packed nanoparticles supported on flat hexagonal, square and trigonal lattices
The tailored design of nanoparticles becomes more important with the advancement of heterogeneous catalysis and materials science. The formation of nanoparticles in catalysts with a specific geometry of the active site becomes necessary to improve activity and selectivity in catalysis. Here we have used physical sphere-in-contact models of various nanoparticles with hexagonal, square and trigonal geometries on flat close-packed surfaces to understand how the distribution of (100) and (111) sites changes as a function of nanoparticle (NP) size in a simplified model of nanoparticle supported metals. The results from this approach clearly show that in 2-layer NPs that have a hexagonal base have 2–3 times more (100) sites than the square and trigonal base NPs as a function of the number of atoms in the NP. In 3D isotropic NPs, this phenomenon is even more pronounced than the 2-layer NPs. We derive equations that estimate the number of (100), (111), the number of atoms and the aspect ratio as a function of n. These equations are important in tailoring the properties of NPs supported on close-packed metal surfaces, which may find applications in materials science, nanotechnology and catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


