通过炔烃与硝基烯烃的还原加氢反应进行金催化胺合成
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胺是医药化合物中最重要的一类有机基团。在此,我们提供了一个通过 Au-H/Au+/Au-H 三重级联催化实现催化剂分化的胺类化合物通用合成蓝图。母催化剂被分化成一组催化活性物种,从而实现三重级联催化,其中每个催化物种都针对一个催化循环进行了专门调整。这种策略可以通过炔烃与硝基烯烃的还原加氢反应合成与生物相关的胺基团。利用这种三重级联方法,我们实现了优异的官能团耐受性,能够使用大宗化学原料作为偶联剂,对简单和复杂的炔烃进行胺化(100 个实例),包括从药物、肽和天然产品中提取的炔烃(30 个实例)。氢化物金和氢化物桥接金络合物的分离和完整晶体学表征,使我们对基本有机金属氢化物金络合物的催化剂分化过程有了更深入的了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
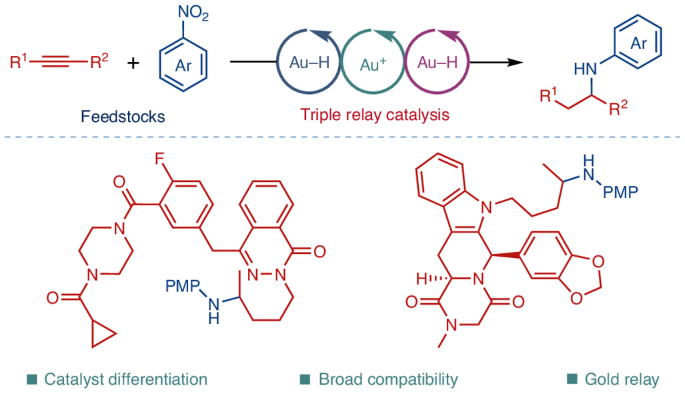
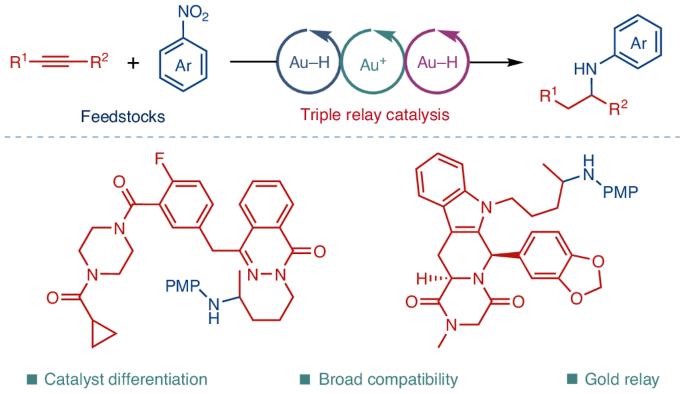
Gold-catalysed amine synthesis by reductive hydroamination of alkynes with nitroarenes
Amines are the most pivotal class of organic motifs in pharmaceutical compounds. Here we provide a blueprint for a general synthesis of amines by catalyst differentiation enabled by triple Au–H/Au+/Au–H relay catalysis. The parent catalyst is differentiated into a set of catalytically active species to enable triple cascade catalysis, where each catalytic species is specifically tuned for one catalytic cycle. This strategy enables the synthesis of biorelevant amine motifs by reductive hydroamination of alkynes with nitroarenes. Using this triple cascade approach, we have achieved exceptional functional group tolerance, enabling the use of bulk chemical feedstocks as coupling partners for the amination of both simple and complex alkynes (>100 examples), including those derived from pharmaceuticals, peptides and natural products (>30 examples). The isolation and full crystallographic characterization of gold hydride and hydride-bridged gold complexes has garnered insights into the catalyst differentiation process of fundamental organometallic gold hydride complexes. Amines are predominant motifs in pharmaceuticals, but complex amines are challenging to generate. Now, enabled by triple Au–H/Au+/Au–H relay catalysis, the synthesis of complex and structurally diverse amines by a direct reductive hydroamination of alkynes with nitroarenes is reported. Catalytic intermediates were isolated to elucidate the mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


