银催化烯烃的 1,2-硫代磺酰化反应:开发亲核 d3-甲硫基试剂
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在药物发现领域,开发稳健的 d3-甲硫基试剂是获得氚代分子的一种极具吸引力的合成方法。在此,我们报告了一种无需柱纯化、一步制备亲电 S-甲基-d3 芳基硫代磺酸酯的直接策略。这些试剂在银催化下对水中的烯烃或 1,6- 烯炔进行邻接硫代磺酰化时表现出良好的自由基反应活性。因此,在不饱和碳碳键中同时加入 SCD3 和 ArSO2 单元的原子经济性达到了 100%。此外,具有 >99% D 结合率的 ATRA 加合物可以在温和的碱性条件下通过逆迈克尔加成法成为亲核的 d3-甲硫基合成物,为三氘代甲基硫代烷基碘化物的高效获取相应的三氘代甲基硫化物提供了一种良好的替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
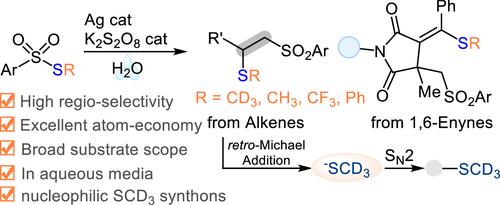
Silver-Catalyzed 1,2-Thiosulfonylation of Alkenes: Development of a Nucleophilic d3-Methylthiolating Reagent
Development of robust d3-methylthiolating reagents represents an attractive synthetic method to access deuterated molecules in the field of drug discovery. Here, we report a straightforward strategy to prepare electrophilic S-methyl-d3 arylsulfonothioates in one-step without column purification. These reagents exhibit good radical reactivity toward silver-catalyzed vicinal thiosulfonylation of alkenes or 1,6-enynes on water. As a result, simultaneous incorporation of both SCD3 and ArSO2 units into unsaturated carbon–carbon bonds with 100% atom economy has been established. Moreover, the ATRA adducts with >99% D incorporation can serve as nucleophilic d3-methylthiolating synthons via retro-Michael addition under mild basic conditions, providing a good alternative in trideuteromethylthiolating alkyl iodides to access corresponding trideuteromethyl sulfides with high efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


