食用海藻中提取的碘酪氨酸氯化过程中的反应性、途径和碘化消毒副产物的形成
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
在家庭烹饪过程中,从可食用海藻中提取的碘会显著增强碘消毒副产物(I-DBPs)的形成。研究了氯与海藻中提取的一碘酪氨酸(MIT)和二碘酪氨酸(DIT)的反应。计算得出次氯酸与中性和阴离子 MIT 反应的特定物种二阶速率常数(25 °C)分别为 23.87 ± 5.01 和 634.65 ± 75.70 M-1 s-1,而与中性和阴离子 DIT 反应的相应速率常数分别为 12.51 ± 19.67 和 199.12 ± 8.64 M-1 s-1。温度的升高促进了氯与 MIT 和 DIT 的反应。根据 HPLC/Q-Orbitrap HRMS 对 59 种碘酪氨酸转化产物/DBP 的鉴定,提出了三种主要的反应途径。利用密度泛函理论进行计算建模的热力学结果表明,卤素交换反应遵循逐步加成-消除的途径。在这些 DBPs 中,3,5-二碘-4-羟基苯甲醛和 3,5-二碘-4-羟基苯乙腈具有较高的毒性风险。在 MIT 和 DIT 的氯化过程中,碘化三卤甲烷和卤乙酸成为普通烹饪温度(80 °C)下的主要物种。这些结果让人们深入了解了卤素交换反应的机理,并对家庭烹饪含碘食物时接触 I-DBPs 的毒性风险产生了重要影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
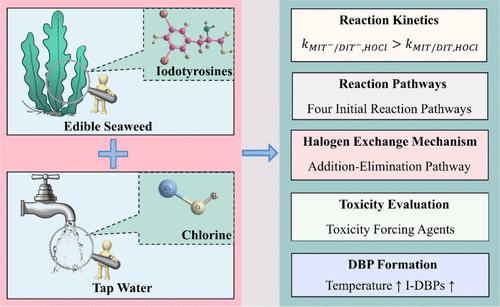
Reactivity, Pathways, and Iodinated Disinfection Byproduct Formation during Chlorination of Iodotyrosines Derived from Edible Seaweed
Iodine derived from edible seaweed significantly enhances the formation of iodinated disinfection byproducts (I-DBPs) during household cooking. Reactions of chlorine with monoiodotyrosine (MIT) and diiodotyrosine (DIT) derived from seaweed were investigated. Species-specific second-order rate constants (25 °C) for the reaction of hypochlorous acid with neutral and anionic MIT were calculated to be 23.87 ± 5.01 and 634.65 ± 75.70 M–1 s–1, respectively, while the corresponding rate constants for that with neutral and anionic DIT were determined to be 12.51 ± 19.67 and 199.12 ± 8.64 M–1 s–1, respectively. Increasing temperature facilitated the reaction of chlorine with MIT and DIT. Based on the identification of 59 transformation products/DBPs from iodotyrosines by HPLC/Q-Orbitrap HRMS, three dominant reaction pathways were proposed. Thermodynamic results of computational modeling using density functional theory revealed that halogen exchange reaction follows a stepwise addition–elimination pathway. Among these DBPs, 3,5-diiodo-4-hydroxy-benzaldehyde and 3,5-diiodo-4-hydroxy-benzacetonitrle exhibited high toxic risk. During chlorination of MIT and DIT, iodinated trihalomethanes and haloacetic acids became dominant species at common cooking temperature (80 °C). These results provide insight into the mechanisms of halogen exchange reaction and imply important implications for the toxic risk associated with the exposure of I-DBPs from household cooking with iodine-containing food.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


