用甲酸和其他氧气清除剂增强以过氧化氢为燃料的活性粒子的自推进运动
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们报告说,通过添加低浓度的氧清除剂,如甲酸(以及其他有机酸、肼和柠檬酸),过氧化氢燃料自扩散活性粒子系统中的活性粒子运动增强了 400%,而高浓度的活性粒子运动则受到抑制。对照实验表明,运动的增强与过氧化氢的催化分解率无关,对颗粒表面化学性质也不敏感。实验结果表明,大量氧清除是活性运动增强的原因,实现了最近预测的产物汇对自扩散运动的促进作用。在氧清除剂浓度较高时,活性运动减弱,这是因为吸附溶质阻塞了催化位点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
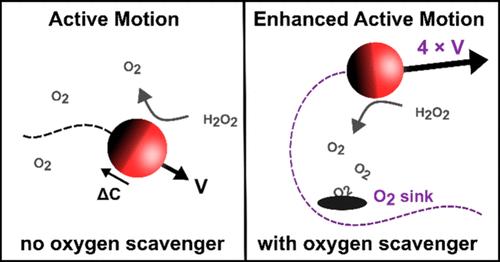
Enhancing the Self-Propelled Motion of Hydrogen Peroxide Fueled Active Particles with Formic Acid and Other Oxygen Scavengers
We report enhanced active particle motion in hydrogen peroxide-fueled self-diffusiophoretic active particle systems of up to 400% via addition of low concentrations of oxygen scavenging agents such as formic acid (as well as other organic acids, hydrazine, and citric acid), whereas active motion was inhibited at higher concentrations. Control experiments showed that enhanced motion was decoupled from catalytic hydrogen peroxide decomposition rate and insensitive to particle surface chemistry. Experimental results point to bulk oxygen scavenging as the cause for the enhanced active motion, representing a realization of recently predicted promotional effects of product sinks on self-diffusiophoretic motion. Diminished active motion at high oxygen scavenger concentrations was attributed to catalytic site blocking by adsorbed solute.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


