从假单胞菌中发现非典型 C16-三萜类化合物并进行生物合成
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
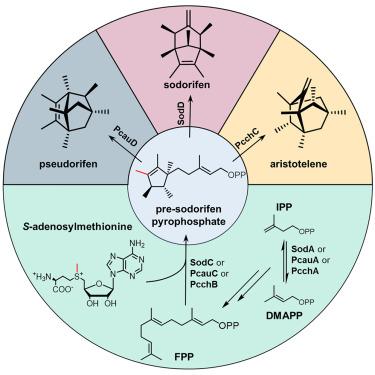
具有独特 C16-双环[3.2.1]辛烯框架的索多里芬的生物合成需要依赖 S-腺苷蛋氨酸的甲基转移酶 SodC 和萜烯环化酶 SodD。虽然生物信息学分析表明 SodCD 基因在细菌中广泛分布,但它们的功能多样性在很大程度上仍然未知。在本文中,来自假单胞菌的两个 sodorifen 型基因簇 pcch 和 pcau 在大肠杆菌中进行了异源表达,从而发现了两种 C16 类萜类化合物。这两种(类似 SodCD)途径特异性酶实现了这些化合物的酶合成。利用 pcch、pcau 和 sod 途径中不同组合的甲基转移酶和萜烯合成酶进行的酶测定揭示了一种统一的生物合成机制:所有三种 SodC 样酶都将焦磷酸法尼酯(FPP)甲基化,随后环化成一种共同的中间体--焦磷酸前索多瑞芬。这种联合前体的结构多样化仅通过随后作用的单个萜烯合成酶来实现。我们的发现拓展了对不常见的 C16-萜类化合物的基本生物合成认识和结构多样性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Discovery and biosynthesis of non-canonical C16-terpenoids from Pseudomonas
Biosynthesis of sodorifen with a unique C16-bicyclo[3.2.1]octene framework requires an S-adenosyl methionine-dependent methyltransferase SodC and terpene cyclase SodD. While bioinformatic analyses reveal a wide distribution of the sodCD genes organization in bacteria, their functional diversity remains largely unknown. Herein, two sodorifen-type gene clusters, pcch and pcau, from Pseudomonas sp. are heterologously expressed in Escherichia coli, leading to the discovery of two C16 terpenoids. Enzymatic synthesis of these compounds is achieved using the two (SodCD-like) pathway-specific enzymes. Enzyme assays using different combinations of methyltransferases and terpene synthases across the pcch, pcau, and sod pathways reveal a unifying biosynthetic mechanism: all three SodC-like enzymes methylate farnesyl pyrophosphate (FPP) with subsequent cyclization to a common intermediate, pre-sodorifen pyrophosphate. Structural diversification of this joint precursor solely occurs by the subsequently acting individual terpene synthases. Our findings expand basic biosynthetic understanding and structural diversity of unusual C16-terpenoids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


