代谢调节因子 PKM2 的缺乏会激活磷酸戊糖通路并产生 TCF1+ 祖 CD8+ T 细胞,从而改善免疫疗法
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
TCF1高的祖CD8+ T细胞可介导免疫疗法的疗效;然而,人们对其产生和维持的机制知之甚少。在这里,我们发现通过删除丙酮酸激酶肌2(PKM2)来靶向糖酵解会导致磷酸戊糖途径(PPP)活性升高,从而富集类似TCF1高祖细胞耗竭的表型,并增加体内对PD-1阻断的反应性。PKM2KO CD8+ T细胞显示出糖酵解通量降低、糖酵解中间产物和PPP代谢产物积累以及1,2-13C葡萄糖碳追踪测定的PPP循环增加。在没有急性糖酵解损伤的情况下,小分子激动剂激动 PPP 可使 CD8+ T 细胞向 TCF1 高的群体倾斜,产生独特的转录景观,激动剂处理过的 CD8+ T 细胞与 PD-1 阻断联合应用可增强对小鼠肿瘤的控制,并促进患者来源的肿瘤组织细胞对肿瘤的杀伤。我们的研究证明了一种新的代谢重编程,有助于形成一种促进免疫疗法疗效的祖细胞样 T 细胞状态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
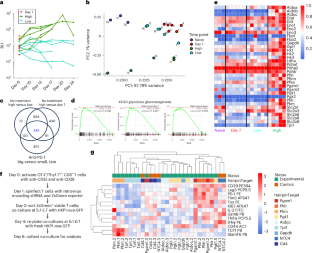
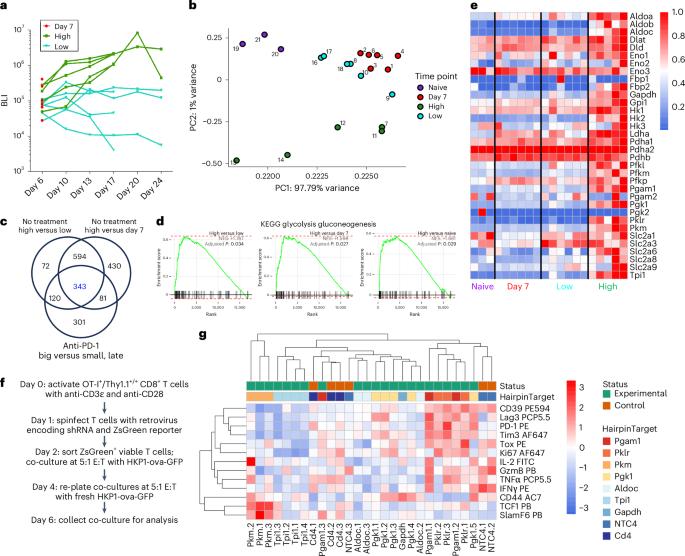
Deficiency of metabolic regulator PKM2 activates the pentose phosphate pathway and generates TCF1+ progenitor CD8+ T cells to improve immunotherapy
TCF1high progenitor CD8+ T cells mediate the efficacy of immunotherapy; however, the mechanisms that govern their generation and maintenance are poorly understood. Here, we show that targeting glycolysis through deletion of pyruvate kinase muscle 2 (PKM2) results in elevated pentose phosphate pathway (PPP) activity, leading to enrichment of a TCF1high progenitor-exhausted-like phenotype and increased responsiveness to PD-1 blockade in vivo. PKM2KO CD8+ T cells showed reduced glycolytic flux, accumulation of glycolytic intermediates and PPP metabolites and increased PPP cycling as determined by 1,2-13C glucose carbon tracing. Small molecule agonism of the PPP without acute glycolytic impairment skewed CD8+ T cells toward a TCF1high population, generated a unique transcriptional landscape and adoptive transfer of agonist-treated CD8+ T cells enhanced tumor control in mice in combination with PD-1 blockade and promoted tumor killing in patient-derived tumor organoids. Our study demonstrates a new metabolic reprogramming that contributes to a progenitor-like T cell state promoting immunotherapy efficacy. TCF1+ progenitor-exhausted CD8+ T cell populations mediate durable antitumor immunity. Upon antigenic stimulation, effector T cells upregulate aerobic glycolysis to support their cytotoxic phenotype. Here, Mittal and colleagues find that loss of metabolic regulator PKM2 enriches for TCF1+ progenitor-exhausted-like cells and improves responsiveness to PD-1 blockade.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


