通过立体选择性异构 O-烷基化合成 2,3-二叠氮-2,3-二脱氧-β-d-甘露糖苷和 2,3-二叠氮-2,3-二脱氧-β-d-甘露糖酸
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
通过 Cs2CO3 介导的 2,3-二叠氮-2,3-二脱氧-β-d-甘露糖与初级亲电体的 O-烷基化反应,完成了 2,3-二叠氮-2,3-二脱氧-β-d-甘露糖的立体选择性合成。对 2,3-二叠氮-2,3-二脱氧-β-d-甘露糖苷的 C6 伯醇进行选择性氧化,成功地生成了相应的 2,3-二叠氮-2,3-二脱氧-β-d-甘露糖酸。本文章由计算机程序翻译,如有差异,请以英文原文为准。
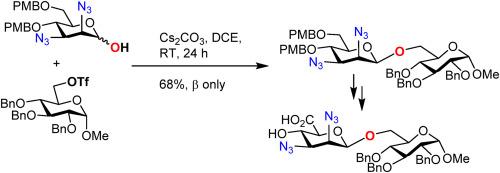
Synthesis of 2,3-diazido-2,3-dideoxy-β-d-mannosides and 2,3-diazido-2,3-dideoxy-β-d-mannuronic acid via stereoselective anomeric O-alkylation
Stereoselective synthesis of 2,3-diazido-2,3-dideoxy-β-d-mannosides has been accomplished via Cs2CO3-mediated anomeric O-alkylation of 2,3-diazido-2,3-dideoxy-β-d-mannoses with primary electrophiles. Selective oxidation of the C6 primary alcohol of the 2,3-diazido-2,3-dideoxy-β-d-mannoside successfully produced corresponding 2,3-diazido-2,3-dideoxy-β-d-mannuronic acid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


