高效合成八种立体异构体的亚氨基糖--灯盏花苷和 1,4-二脱氧-1,4-亚氨基-D-阿拉伯糖醇 (DAB)
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在此,我们介绍了以 4-苯甲酰基-6-脱氧-6-碘甘吡喃糖苷 47 为起始原料,高效非对映选择性合成 1,4-二脱氧-1,4-亚氨基-D-阿拉伯糖醇(DAB)1b、灯盏花苷 3a、以及这几种亚氨基糖的七种立体异构体,以 4-苯甲酰基-6-脱氧-6-碘甘油吡喃糖苷 47 为起点,DAB 和异构体 1a-1h 的产率为 38 % 至 68 %,灯盏花苷和异构体 3a-3h 的产率为 44 % 至 89 %。我们还报告了利用市售糖合成 4-苯甲酰基-6-脱氧-6-碘甘吡喃糖苷 47 的八种立体异构体的情况。亚氨基糖合成的关键是一个单一的多步反应,通过锌介导的一锅还原消除反应将 4-苯甲酰基-6-脱氧-6-碘甘氨酰吡喃糖苷 47 转化为乙烯基吡咯烷,然后进行还原胺化反应,最后进行分子内亲核取代反应。战略性地选择还原胺化过程中使用的胺以及中间碳碳双键的官能化,可以获得大量的亚氨基糖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
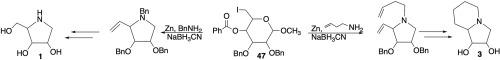
Efficient synthesis for each of the eight stereoisomers of the iminosugars lentiginosine and 1,4-dideoxy-1,4-imino-D-arabinitol (DAB)
Herein, we describe the efficient, diastereoselective syntheses of the iminosugars 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) 1b, lentiginosine 3a, and the seven stereoisomers of each of these iminosugars starting from 4-benzoyl-6-deoxy-6-iodoglycopyranosides 47 with yields ranging from 38 % to 68 % for the DAB and isomers 1a-1h and from 44 % to 89 % for the lentiginosine and isomers 3a-3h. We also report the syntheses of the eight stereoisomers of the 4-benzoyl-6-deoxy-6-iodoglycopyranosides 47 from commercially available sugars. Key to the iminosugar syntheses is a single multistep reaction that converts the 4-benzoyl-6-deoxy-6-iodoglycopyranosides 47 to a vinyl pyrrolidine through a one-pot zinc mediated reductive elimination, followed by a reductive amination and finally an intramolecular nucleophilic substitution. Strategic selection of the amine utilized in the reductive amination and the functionalization of the intermediate carbon-carbon double bond provides access to a vast array of iminosugars.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


