合理设计 Er2O3/ZnS 作为高能电催化剂在 OER 中的应用
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
为了改善缓慢的氧进化反应(OER),需要制造一种产量极高、成本低廉且性能突出的电催化剂。本文采用水热法制备了 Er2O3/ZnS 复合材料,作为高效水分离的电极材料。报告中使用了各种分析工具来评估材料的形态、结晶度、功能性、表面积和热稳定性。Er2O3/ZnS 复合材料的比表面积较大(61.06 m2 g-1),使其成为进行 OER 过程的合适候选材料。此外,还在泡沫镍(NF)基底上对 Er2O3/ZnS 复合材料进行了电化学研究,以确定其电解性质。电化学研究表明,在 10 mA cm-2 的理想电流密度 (j) 下,合成的复合材料具有显著的过电位(260 mV)和较低的塔菲尔值(40 mV dec-1)。电化学观察结果表明,将 Er2O3 加入 ZnS 后,表面积增大,活性区域增强,从而降低了电阻,促进了电解质离子的快速结合。利用这种方法开发的复合材料(Er2O3/ZnS)可用于各种能量转换和储存应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
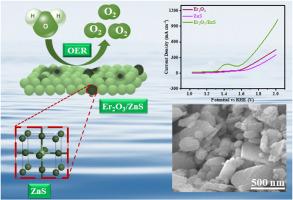
Rational design of Er2O3/ZnS as highly competent electrocatalyst for OER application
The fabrication of an extremely productive, inexpensive and prominent electrocatalyst is required to improve the slow oxygen evolution reaction (OER). Here, a hydrothermal approach was utilized to fabricate Er2O3/ZnS composite as a competent electrode material for the purpose of efficient water splitting. The various analytical tools were used to evaluate morphology, crystallinity, functionality, surface area, and thermal stability of the reported materials. The large surface area of the Er2O3/ZnS composite (61.06 m2 g−1), makes it a suitable candidate for carrying out the OER process. Moreover, the electrochemical study for Er2O3/ZnS composite was conducted on nickel foam (NF) as a substrate to determine the electrolytic nature. The electrochemical study showed that the synthesized composite responds to an impressive overpotential (260 mV) and low Tafel value (40 mV dec−1) at an ideal current density (j) of 10 mA cm−2. The Er2O3/ZnS composite exhibits a reduced onset potential of approximately 1.31 V and exceptional durability of about 30 h. The electrochemical observation suggests that incorporating Er2O3 into ZnS resulted in an enlarged surface area and enhanced active regions which reduce the resistance and promote the rapid binding of electrolyte ions. The composite (Er2O3/ZnS) developed by this approach can be utilized in various energy conversion and storage applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


