自组装的 PROTACs 可使蛋白质降解,从而重新规划肿瘤微环境,协同增强结直肠癌免疫疗法
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
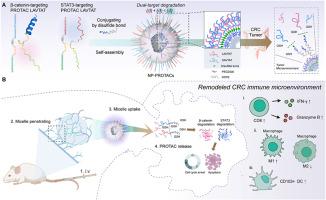
β-catenin和STAT3共同驱动结直肠癌(CRC)的生长、进展和免疫逃避,它们的共同表达与不良预后密切相关。然而,由于STAT3和β-catenin之间存在相互反馈激活作用,目前的小分子抑制剂疗效有限。受PROteolysis TArgeting Chimera(PROTAC)这种选择性降解蛋白质的药物学模式的启发,我们开发了一种纳米工程肽PROTACs(NP-PROTACs)策略,以有效降解β-catenin和STAT3。NP-PROTACs 是通过二硫键将多肽 PROTACs 与 DSPE-PEG 连接并自组装成纳米颗粒。值得注意的是,与单靶点治疗相比,NP-PROTACs介导的β-catenin和STAT3双重降解产生了协同抗肿瘤效应。此外,NP-PROTACs还能增强CD103+树突状细胞浸润和T细胞细胞毒性,缓解β-catenin/STAT3诱导的CRC免疫抑制微环境。这些结果凸显了 NP-PROTACs 在促进同时降解两种致病蛋白方面的潜力,从而为癌症治疗提供了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Self-assembled PROTACs enable protein degradation to reprogram the tumor microenvironment for synergistically enhanced colorectal cancer immunotherapy
Both β-catenin and STAT3 drive colorectal cancer (CRC) growth, progression, and immune evasion, and their co-overexpression is strongly associated with a poor prognosis. However, current small molecule inhibitors have limited efficacy due to the reciprocal feedback activation between STAT3 and β-catenin. Inspired by the PROteolysis TArgeting Chimera (PROTAC), a promising pharmacological modality for the selective degradation of proteins, we developed a strategy of nanoengineered peptide PROTACs (NP-PROTACs) to degrade both β-catenin and STAT3 effectively. The NP-PROTACs were engineered by coupling the peptide PROTACs with DSPE-PEG via disulfide bonds and self-assembled into nanoparticles. Notably, the dual degradation of β-catenin and STAT3 mediated by NP-PROTACs led to a synergistic antitumor effect compared to single-target treatment. Moreover, NP-PROTACs treatment enhanced CD103+ dendritic cell infiltration and T-cell cytotoxicity, alleviating the immunosuppressive microenvironment induced by β-catenin/STAT3 in CRC. These results highlight the potential of NP-PROTACs in facilitating the simultaneous degradation of two pathogenic proteins, thereby providing a novel avenue for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


