角质细胞整合素 α3β1 促进伤口表皮的有效愈合
JID innovations : skin science from molecules to population health
Pub Date : 2024-08-29
DOI:10.1016/j.xjidi.2024.100310
引用次数: 0
摘要
迄今为止,有关表皮整合素α3β1在皮肤伤口再上皮化中的作用的研究得出了相互矛盾的结果:对全基因α3缺失的新生小鼠皮肤伤口的研究表明,整合素能促进伤口及时再上皮化,而对组成型表皮特异性α3β1缺失的成年小鼠的研究则没有发现这种作用。本研究的目的是利用表皮中可诱导的α3β1缺失模型来阐明α3β1在成人伤口愈合中的作用。我们利用最近开发的转基因K14Cre-ERT::α3flx/flx小鼠(即诱导性α3表皮基因敲除),在使用4-羟基他莫昔芬局部处理伤口之前,从表皮中删除浮性Itga3等位基因(α3flx/flx)。这样就可以阐明成年皮肤中α3β1依赖性伤口愈合的情况,而不需要考虑广泛使用的组成型α3β1基因敲除小鼠胚胎期缺失表皮α3β1后可能出现的代偿机制。我们发现,诱导性α3表皮基因敲除小鼠的伤口再表皮化间隙比对照小鼠大,表明伤口愈合延迟,表皮整合素α3β1至少部分通过增强角质形成细胞的增殖来促进伤口愈合。这项工作为今后研究将整合素α3β1作为促进伤口愈合的治疗靶点提供了重要依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
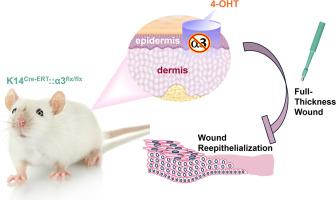
Keratinocyte Integrin α3β1 Promotes Efficient Healing of Wound Epidermis
To date, studies of the role for epidermal integrin α3β1 in cutaneous wound re-epithelialization have produced conflicting results: wound studies in skin from global α3-null neonatal mice have implicated the integrin in promoting timely wound re-epithelialization, whereas studies in adult mice with constitutive, epidermal-specific α3β1 deletion have not. The objective of this study was to utilize a model of inducible α3β1 deletion in the epidermis to clarify the role of α3β1 in the healing of adult wounds. We utilized the recently developed transgenic K14Cre-ERT::α3flx/flx mice (ie, inducible α3 epidermal knockout), permitting us to delete floxed Itga3 alleles (α3flx/flx) from epidermis just prior to wounding with topical treatment of 4-hydroxytamoxifen. This allows for the elucidation of α3β1-dependent wound healing in adult skin, free from compensatory mechanisms that may occur after embryonic deletion of epidermal α3β1 in the widely used constitutive α3β1-knockout mouse. We found that re-epithelializing wound gaps are larger in inducible α3 epidermal knockout mice than in control mice, indicating delayed healing, and that epidermal integrin α3β1 promotes healing of wounds, at least in part by enhancing keratinocyte proliferation. This work provides essential rationale for future studies to investigate integrin α3β1 as a therapeutic target to facilitate wound healing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.00
自引率
0.00%
发文量
0
审稿时长
8 weeks

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


