1H-1-羟基喹啉-4-酮的高选择性电合成--合成多功能天然抗生素的途径
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
1H-1- 羟基喹啉-4-酮是一类具有广泛生物活性的杂环化合物,具有外环 N、O 基团。电合成法提供了通过硝基还原获得 1-羟基喹啉-4-酮的直接、高选择性和可持续的途径。从容易获得的 2-硝基苯甲酸开始,建立了一条多功能合成路线。在 26 个实例中证明了该方法的广泛适用性,收率高达 93%,其中以天然抗生素 Aurachin C 和 HQNO 为例最为突出。多克级电解法突出了该方法的实用性和技术相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
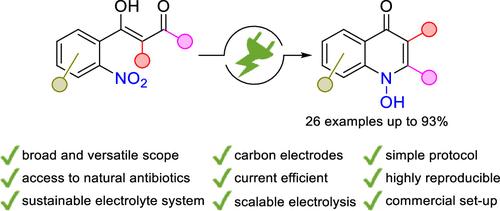
Highly Selective Electrosynthesis of 1H-1-Hydroxyquinol-4-ones–Synthetic Access to Versatile Natural Antibiotics
1H-1-Hydroxyquinolin-4-ones represent a broad class of biologically active heterocycles having an exocyclic N,O motif. Electrosynthesis offers direct, highly selective, and sustainable access to 1-hydroxyquinol-4-ones by nitro reduction. A versatile synthetic route starting from easily accessible 2-nitrobenzoic acids was established. The broad applicability of this protocol was demonstrated on 26 examples with up to 93% yield, highlighted by the naturally occurring antibiotics Aurachin C and HQNO. The practicability and technical relevance were underlined by multigram scale electrolysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


