碳-碳单电子σ键的直接证据
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
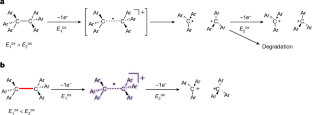
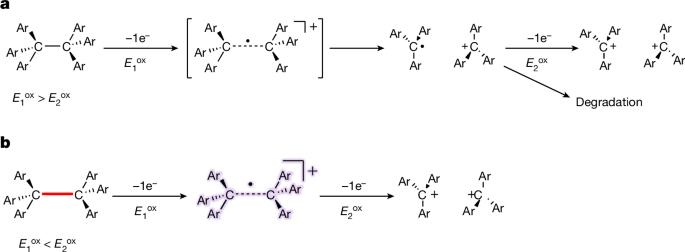
共价键在两个原子间共享电子对,并以单键、双键和三键的形式构成大多数有机化合物的骨架。相比之下,单电子键的例子仍然很少,这很可能是由于其固有的弱点1,2,3,4。尽管有几项开创性的研究报告了杂原子间的单电子键,但碳原子间单电子键的直接证据仍然难以找到。在此,我们报告了通过对具有拉长 C-C 单键的碳氢化合物进行单电子氧化,分离出一种碳原子间具有单电子σ键的化合物5,6。单晶 X 射线衍射分析和拉曼光谱实验证实了 C-C 单电子σ键(100 K 时为 2.921(3) Å)的存在,密度泛函理论计算也证实了这一点。本文的研究结果明确证明了近一个世纪前提出的 C-C 单电子σ键的存在7 ,从而有望通过探测成键态与非成键态之间的边界,为化学不同领域的进一步发展铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Direct evidence for a carbon–carbon one-electron σ-bond
Covalent bonds share electron pairs between two atoms and make up the skeletons of most organic compounds in single, double and triple bonds. In contrast, examples of one-electron bonds remain scarce, most probably due to their intrinsic weakness1–4. Although several pioneering studies have reported one-electron bonds between heteroatoms, direct evidence for one-electron bonds between carbon atoms remains elusive. Here we report the isolation of a compound with a one-electron σ-bond between carbon atoms by means of the one-electron oxidation of a hydrocarbon with an elongated C–C single bond5,6. The presence of the C•C one-electron σ-bond (2.921(3) Å at 100 K) was confirmed experimentally by single-crystal X-ray diffraction analysis and Raman spectroscopy, and theoretically by density functional theory calculations. The results of this paper unequivocally demonstrate the existence of a C•C one-electron σ-bond, which was postulated nearly a century ago7, and can thus be expected to pave the way for further development in different areas of chemistry by probing the boundary between bonded and non-bonded states. The one-electron oxidation of a hydrocarbon with an elongated C–C single bond provides direct evidence for a one-electron σ-bond between carbon atoms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


