首次揭示氧化还原变化过程中岩溶土壤中砷的迁移和螯合作用
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
岩溶地貌为地球上约 25% 的人口提供饮用水,尤其是在中国西南地区。土壤中的砷等污染物会通过岩溶地貌中的天坑和基岩裂缝渗入地下水。尽管如此,岩溶土壤在氧化还原变化条件下释放砷的内在机制在很大程度上仍未得到研究。在这里,我们使用多种同步辐射光谱分析方法,在模拟实地验证的氧化还原条件下,探索砷污染岩溶土壤中砷的迁移和固碳。我们观察到,土壤中的砷主要以砷(V)的形式存在,而砷(V)主要与铁氧水氧化物伴生。溶解的 As 浓度很高(294 μM),在低 Eh(≤-100 mV)条件下,As(III) 占主导地位(∼95%),这表明在还原条件下 As 浸出的风险很高。这种 As 迁移是由于铁水物和方解石的溶解促进了相关 As(V) 的释放和还原。在高 Eh(≥+100 mV)条件下,溶解的 As 浓度较低(17.0 μM),As(V)占优势(∼68%),这可能是由于再结晶的铁相氧化和/或封存了 As(III)。我们的研究结果表明,铁相和非铁相还原释放 As(V)以及铁相再结晶的共同作用,决定了岩溶环境土壤中氧化还原诱导的砷迁移和潜在浸出。本文章由计算机程序翻译,如有差异,请以英文原文为准。
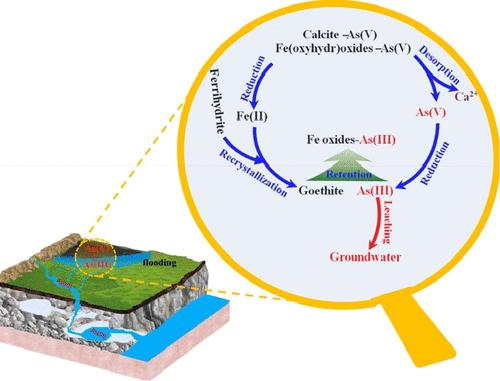
First Insight into the Mobilization and Sequestration of Arsenic in a Karstic Soil during Redox Changes
Karst terrains provide drinking water for about 25% of the people on our planet, particularly in the southwest of China. Pollutants such as arsenic (As) in the soil can infiltrate groundwater through sinkholes and bedrock fractures in karst terrains. Despite this, the underlying mechanisms responsible for As release from karst soils under redox changes remain largely unexplored. Here, we used multiple synchrotron-based spectroscopic analyses to explore As mobilization and sequestration in As-polluted karstic soil under biogeochemical conditions that mimic field-validated redox conditions. We observed that As in the soil exists primarily as As(V), which is mainly associated with Fe(oxyhydr)oxides. The concentration of the dissolved As was high (294 μM) and As(III) was dominant (∼95%) at low Eh (≤−100 mV), indicating the high risk of As leaching under reducing conditions. This As mobilization was attributed to the fact that the dissolution of ferrihydrite and calcite promoted the release and reduction of associated As(V). The concentration of the dissolved As was low (17.0 μM) and As(V) was dominant (∼68%) at high Eh (≥+100 mV), which might be due to the oxidation and/or sequestration of As(III) by the recrystallized ferric phase. Our results showed that the combined effects of the reductive release of As(V) from both ferric and nonferric phases, along with the recrystallization of the ferric phase, govern the redox-induced mobilization and potential leaching of As in soils within karst environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


