基于 Co、Zn 驱动的双锚定自催化 Ru 再沉积,实现稳健的酸性水氧化作用
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
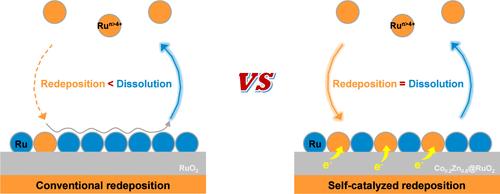
由于与基质的电子相互作用较弱,高价位的 Run>4+ 在氧化电位下的再沉积速度比其溶解速度慢,从而限制了水的氧化性能。我们提出了一种由 Co 和 Zn 共掺助推的新型自催化再沉积策略,以建立新的 Ru 溶解-再沉积平衡。掺杂 Co、Zn 的 RuO2 具有独特的双重锚定效应,其中电子负载的 Co 和 Zn 可通过抑制过氧化和缩短 Ru-O 键(第一锚定)来减少 Run>4+ 的沥滤。更重要的是,通过 Co2+/Co3+ 氧化还原驱动的自催化作用,Run>4+ 可以快速稳定地沉积为活性 Ru(OH)62-(第二锚定)。调节 Co 的含量可以调节自催化的强度,从而通过从 Co 到 Ru 的定向电子流稳定溶解-再沉积平衡。再沉积的 Co0.2Zn0.8@RuO2 显示出缩短的 Ru-O 键和丰富的缺陷,在 10 mA-cm-2 (150 mV) 时显示出超低的过电位,超过了 RuO2 基准和大多数催化剂。此外,Co0.2Zn0.8@RuO2 在 500 mA-cm-2 下可持续 100 小时,表现出卓越的稳定性。这种自催化再沉积为打破电催化剂的活性-稳定性权衡提供了一种新的方法,尤其是在膜水电解槽中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Self-Catalyzed Ru Redeposition Based on Co, Zn-Driven Double Anchoring for Robust Acidic Water Oxidation
The redeposition of high-valence Run>4+ at oxidation potential is slower than its dissolution due to weak electronic interactions with substrates, limiting water oxidation performance. We propose a novel self-catalyzed redeposition strategy fuelled by Co and Zn codoping to establish a new dissolution–redeposition balance of Ru. Co, Zn-doped RuO2 with unique double anchoring effect is prepared, where electron-donating Co and Zn reduce Run>4+ leaching by inhibiting overoxidation and shortening Ru–O bonds (first anchoring). More importantly, Run>4+ is rapidly and stably deposited as active Ru(OH)62– through Co2+/Co3+ redox-driven self-catalysis (second anchoring). Regulating the Co content can modulate the self-catalysis strength, thus stabilizing dissolution–redeposition equilibrium via directional electron flow from Co to Ru. The redeposited Co0.2Zn0.8@RuO2 presents shortened Ru–O bonds and abundant defects, displaying ultralow overpotential at 10 mA·cm–2 (150 mV), surpassing the RuO2 benchmark and most catalysts. Furthermore, Co0.2Zn0.8@RuO2 exhibits excellent stability at 500 mA·cm–2 for 100 h. This self-catalyzed redeposition offers a new routine to break the activity–stability trade-off of electrocatalysts, especially in membrane water electrolyzers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


