单位 Mo 改性 SAPO-34 上乙烷向乙烯的光增强选择性转化
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
这项研究揭示了单位钼修饰 SAPO-34 将乙烷选择性转化为乙烯的光增强活性。光照射将乙烷转化为乙烯所需的活化能大幅降低了约 47 kJ/mol。结合结构分析和理论计算,我们确定 HO-Mo(V)=O 是以 C2H5-O-Mo-O(2H) 方式选择性裂解 C2H6 的活性物种。光照射引发电子从双键 O 转变为 Mo,从而促进 C-H 裂解。与此同时,O=Mo(V)-OH 中的光诱导缺电子双键氧充当路易斯酸(LA),而 SAPO-34 的 Al-O-P 中的碱性骨架氧充当路易斯碱(LB),从而形成受挫路易斯对(FLP)。这种合作性相互作用促进了 C-H 键的断裂,并在 LA 和 LB 间距增大的帮助下实现了更有效的 C-H 拉伸。与 Mo-SAPO-34 相比,光助 C-H 活化提高了 C2H4 的整体形成效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
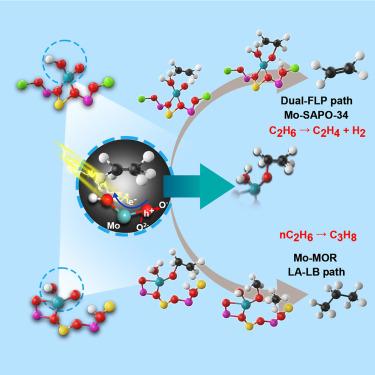
Photo-enhanced selective conversion of ethane to ethene over single-site Mo-modified SAPO-34
This study reveals photo-enhanced activity for selective conversion of ethane to ethene over single-site Mo-modified SAPO-34. Light irradiation significantly reduces the activation energy required for the conversion of ethane to ethene by approximately 47 kJ/mol. Combining structural analyses and theoretical calculations, we identify HO-Mo(V)=O as the active species responsible for the selective C–H cleavage of C2H6 in the manner of C2H5-O-Mo-O(2H). Photo-irradiation triggers the electron transition from the double-bond O to Mo, promoting C–H cleavage. Simultaneously, the photo-induced, electron-deficient, double-bond oxygen in O=Mo(V)-OH acts as the Lewis acid (LA) while the basic skeleton oxygen in Al-O-P of SAPO-34 serves as the Lewis base (LB), creating a frustrated Lewis pair (FLP). This cooperative interaction facilitates the breaking of C–H bonds and allows for more efficient C–H stretching, aided by the increased distance between LA and LB. The photo-assisted C–H activation enhances the overall formation efficiency of C2H4 over Mo-SAPO-34.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


