通过钯催化的分支选择性烯丙基 C-H 烷基化反应对手性 1,4-炔类化合物进行对映选择性合成
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们在此介绍通过钯催化的分支、对映和非对映选择性烯丙基 C-H 烷基化反应,构建具有三级或四级立体中心的手性 1,4-炔。带有笨重取代基的炔基碳似乎表现出了有竞争力的反应性能,所需的手性 1,4-炔以高达 93% 的收率和高达 20:1 b/l、>20:1 dr 和 98% ee 获得。克级规模的实验、苯并噻唑环的可行操作以及制备获得 (+)-Breynolide 和前列腺素的关键中间体,展示了化学合成中的多种合成用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
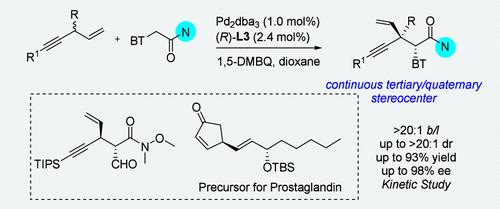
Enantioselective Synthesis of Chiral 1,4-Enynes via Palladium-Catalyzed Branch-Selective Allylic C–H Alkylation
We herein present the construction of a chiral 1,4-enyne featuring tertiary or quaternary stereogenic center via Pd-catalyzed branch-, enantio-, and diastereoselective allylic C–H alkylation. Alkynyl carbon bearing bulky substituents appeared to exhibit competitive reaction performance, and the desired chiral 1,4-enynes were obtained in up to 93% yield and with up to >20:1 b/l, >20:1 dr, and 98% ee. A gram-scale experiment, the feasible operation of benzothiazole ring, and the preparation of the key intermediate to access (+)-Breynolide and prostaglandin are represented as a demonstration of multifarious synthetic utility in chemical synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


