合成 BODIPY-喹啉二元探针,并通过光谱和计算方法研究其与纤维蛋白原/HSA 的生物物理相互作用
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-09-21
DOI:10.1016/j.jphotochem.2024.116047
引用次数: 0
摘要
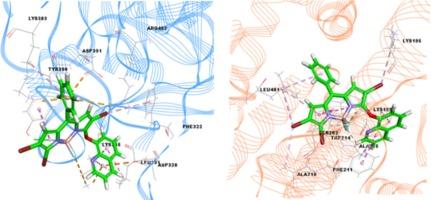
利用稳态/三维(3D)荧光和紫外-可见吸收以及分子对接方法,合成了一种新的基于 BODIPY 的喹啉二元探针(Bdq),并考察了它与纤维蛋白原(Fib)和人血清白蛋白(HSA)的结合亲和力。荧光滴定试验表明,蛋白质的本征发射被 Bdq 以静态和动态相结合的机制淬灭,形成无荧光复合物。三维光谱显示,这两种蛋白质在与 Bdq 作用时发生了构象变化。根据 Förster 的非辐射能量转移 r,发现 Fib/HSA 的结合距离为 3.64/3.47 nm。ΔH(-62.27 kJ/mol)和ΔS(-83.03 J/mol K)参数的负值表明了与 Fib 的范德华力/氢键作用,ΔH(240.0 kJ/mol)和ΔS(920.0 J/molK)参数的正值表明了与 Bdq 的 HSA 的疏水相互作用。分子对接也得出了支持实验结合类型的一致结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis of a BODIPY-quinoline dyad probe and studies of its biophysical interactions with fibrinogen/HSA by spectral and computational methods
A new BODIPY-based quinoline dyad probe (Bdq) was synthesized and its binding affinity with fibrinogen (Fib) and human serum albumin (HSA) was examined using steady-state/three-dimensional (3D) fluorescence and UV–vis absorption as well as molecular docking method. Fluorescence titration assays indicated that protein intrinsic emission was quenched by Bdq with a combined (static and dynamic) mechanism to form a non-fluorescent complex. 3D spectra showed that the two proteins undergo conformational changes when they interact with Bdq. Based on Förster’s non-radiative energy transfer; r, binding distances were found as 3.64/3.47 nm for Fib/HSA. The negative values of both ΔH (−62.27 kJ/mol) and ΔS (−83.03 J/mol K) parameters pointed out van der Waals forces/hydrogen bonds with Fib and also positive values of both ΔH (240.0 kJ/mol) and ΔS (920.0 J/molK) showed hydrophobic interactions with HSA of Bdq. Molecular docking also gave consistent results supporting the experimental binding types.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


