用于水电解槽的具有不同季铵基团的 PPS 增强聚(三联苯)阴离子交换膜
IF 8.4
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
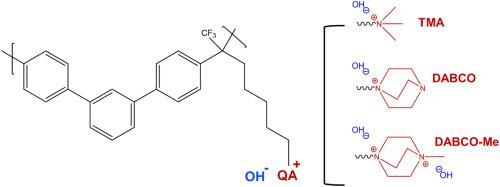
季铵(QA)基团的性质、浓度和空间分布对阴离子交换膜的性能至关重要,主要是通过增强离子交换能力(IEC)。本研究比较了一系列基于聚(偏三联苯)的离子交换膜(mTPN),这些膜所附的季铵基团各不相同。用 1,4-二氮杂双环[2.2.2]辛烷(DABCO)、三甲胺(TMA)和甲基化 DABCO(DABCO-Me)功能化的膜的 IEC 分别为 1.97、2.20 和 3.70 mmol OH- g-1。为了控制膨胀,所有膜都使用聚苯硫醚(PPS)纤维毡进行增强。PPS/mTPN/DABCO-Me 的最高电导率在 1 M KOH 中为 140 mS cm-1,在 4 M KOH 中为 237 mS cm-1。为期 2 个月的碱性稳定性测试表明,在纯水中测量,含有 TMA 和 DABCO 的膜具有稳定的面内电导率,而含有 DABCO-Me 的膜则显示出强烈的电阻增加。另一方面,1 个月的稳定性测试表明,含有 DABCO-Me 的膜在 1 M KOH 中的面内电导率几乎没有变化。这表明,吸收 KOH 溶液的导电性比季铵基团提供的导电性更重要,并有望在电解槽中稳定运行。电解槽测试也证明了这一点,在 0.25 A cm-2 的条件下,测试电压稳定在 1.9 V,在 100 小时的测试时间内(60 °C,4 M KOH,不含 PGM 的催化剂),没有观察到膜电阻的变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PPS-reinforced poly(terphenylene) anion-exchange membranes with different quaternary ammonium groups for use in water electrolysers
The nature, concentration and spatial distribution of quaternary ammonium (QA) groups is instrumental for the performance of anion exchange membranes, primarily by augmenting the ion exchange capacity (IEC). This study compares a series of poly(meta-terphenylene)-based ion exchange membranes (mTPN), which vary in the attached quaternary ammonium group. The IEC of the membranes is 1.97, 2.20 and 3.70 mmol OH− g−1 for membranes functionalized with 1,4-diazabicyclo[2.2.2]octane (DABCO), trimethylamine (TMA), and methylated DABCO (DABCO-Me), respectively. The latter is obtained by methylation of the DABCO-functionalized membrane.
To control swelling, all membranes are reinforced with a poly(phenylene sulfide) (PPS) fiber mat. The highest conductivity, achieved by PPS/mTPN/DABCO-Me, is 140 mS cm−1 in 1 M KOH and 237 mS cm−1 in 4 M KOH. An alkaline stability test over 2 months revealed that membranes with TMA and DABCO have a stable in-plane conductivity, measured in pure water, while the membrane with DABCO-Me showed a strongly increased resistance. On the other hand, a 1 month stability test showed that the through-plane conductivity of the membrane with DABCO-Me in 1 M KOH hardly changed. This indicates that conductivity by absorbed KOH solution is more relevant than the conductivity provided by the quaternary ammonium groups, and promises stable operation in the electrolyser. This is supported by an electrolyser test, where a stable voltage of 1.9 V at 0.25 A cm−2 was achieved, with no observed change in membrane resistance over a test duration of 100 h (60 °C, 4 M KOH, PGM-free catalyst).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Membrane Science
工程技术-高分子科学
CiteScore
17.10
自引率
17.90%
发文量
1031
审稿时长
2.5 months
期刊介绍:
The Journal of Membrane Science is a publication that focuses on membrane systems and is aimed at academic and industrial chemists, chemical engineers, materials scientists, and membranologists. It publishes original research and reviews on various aspects of membrane transport, membrane formation/structure, fouling, module/process design, and processes/applications. The journal primarily focuses on the structure, function, and performance of non-biological membranes but also includes papers that relate to biological membranes. The Journal of Membrane Science publishes Full Text Papers, State-of-the-Art Reviews, Letters to the Editor, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


