改变氟改性 CuIn 双金属材料在电催化二氧化碳还原过程中的优先产物选择性
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
将二氧化碳(CO2)衍生的可再生技术融入石化工业是实现碳中和的一条大有可为的途径。以可再生能源为动力的电化学二氧化碳还原(eCO2R)工艺,特别是使用铜基电催化剂的工艺,是我们为避免气候后果而进行的脱碳努力的宝贵财富。为了提高工艺效率,这项工作是一项独特的案例研究,强调了开发电催化剂与调整操作条件相结合的必要性。本文介绍了一个具有代表性的案例,即使用具有可调 In/Cu 比率的氟改性 CuIn 双金属电催化剂,展示了如何根据反应器配置改变选择性偏好:我们的优化催化剂系统使基于气体扩散电极 (GDE) 和 H 细胞型电解槽的 C2+ 产物的法拉第效率 (FE) 分别达到 68% 和 80%。此外,可控的 In/Cu 比率和氟化物掺杂程度在控制双金属电催化剂的物理化学特性及其 eCO2R 性能方面发挥了关键作用。多种表征工具提供了催化剂结构与性能、反应器配置、电解质和性能之间关系的系统见解,旨在提高 eCO2R 工艺的技术就绪水平。本文章由计算机程序翻译,如有差异,请以英文原文为准。
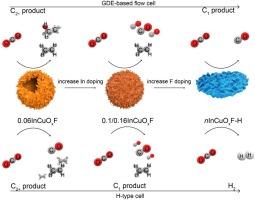
Altering preferential product selectivity in electrocatalytic CO2 reduction over fluorine-modified CuIn bimetallic materials
Integrating carbon dioxide (CO2)-derived renewable technologies into the petrochemical industry presents a promising avenue for carbon neutrality. The renewable-energy-powered electrochemical CO2 reduction (eCO2R) process, particularly over Cu-based electrocatalysts, is a valuable asset for our decarbonization efforts to avoid climate consequences. Intending to enhance process efficiency, this work is a distinctive case study emphasizing the need to develop electrocatalysts in conjunction with adjusting operating conditions. A representative case is presented using fluorine-modified CuIn bimetallic electrocatalysts with tunable In/Cu ratios, demonstrating how to alter selectivity preferences based on reactor configuration: Our optimized catalyst system led to the highest faradaic efficiency (FE) for C2+ products of 68 % and the highest C1 FE of 80 % using gas diffusion electrodes (GDE)- and H-cell-type based electrolyzer, respectively. Moreover, the controllable In/Cu ratio and the degree of fluoride doping played a pivotal role in governing the physicochemical properties of the bimetallic electrocatalysts and their eCO2R performance. Diverse characterization tools offered systematic insights into the relationships between catalyst structure and properties, reactor configuration, electrolyte, and performance, aiming to enhance the Technology Readiness Level of the eCO2R process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


